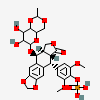
Etopophos
- Etopophos
- [4-[(5S,5aR,8aR,9R)-5-[(7,8-dihydroxy-2-methyl-4,4a,6,7,8,8a-hexahydropyrano[3,2-d][1,3]dioxin-6-yl)oxy]-8-oxo-5a,6,8a,9-tetrahydro-5H-[2]benzofuro[5,6-f][1,3]benzodioxol-9-yl]-2,6-dimethoxyphenyl] dihydrogen phosphate
- 117091-64-2
- SCHEMBL1649994
- AC-421
- Create:2005-08-08
- Modify:2025-01-18
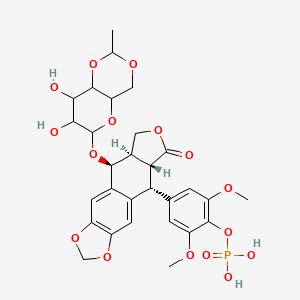
- BMY 40481-30
- BMY-40481
- BMY-40481-30
- Etophos
- Etopofos
- Etopophos
- etoposide phosphate
Etoposide phosphate is approved to be used with other drugs to treat:
• Small cell lung cancer. It is used with cisplatin as first-line therapy.
• Testicular cancer. It is used in patients who have already been treated with surgery, radiation therapy, or other chemotherapy and have not gotten better.
Etoposide phosphate is also available in a different form called etoposide. Etoposide phosphate is also being studied in the treatment of other types of cancer.
Use (kg; approx.) in Germany (2009): >10
Consumption (g per capita; approx.) in Germany (2009): 0.000122
Calculated removal (%): 8.8



H228 (100%): Flammable solid [Danger Flammable solids]
H302 (100%): Harmful if swallowed [Warning Acute toxicity, oral]
H340 (100%): May cause genetic defects [Danger Germ cell mutagenicity]
H350 (100%): May cause cancer [Danger Carcinogenicity]
H360 (100%): May damage fertility or the unborn child [Danger Reproductive toxicity]
H372 (100%): Causes damage to organs through prolonged or repeated exposure [Danger Specific target organ toxicity, repeated exposure]
P203, P210, P240, P241, P260, P264, P270, P280, P301+P317, P318, P319, P330, P370+P378, P405, and P501
(The corresponding statement to each P-code can be found at the GHS Classification page.)
Flam. Sol. 2 (100%)
Acute Tox. 4 (100%)
Muta. 1B (100%)
Carc. 1A (100%)
Repr. 1A (100%)
STOT RE 1 (100%)
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=LIQODXNTTZAGID-GDAYZDJCSA-N
- EU Clinical Trials Register
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-notice630-364-1https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/163461[No public or meaningful name is available]https://echa.europa.eu/substance-information/-/substanceinfo/100.158.659
- NCI Cancer DrugsEtopophos (Etoposide Phosphate)https://www.cancer.gov/about-cancer/treatment/drugs/etoposidephosphate
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/ETOPOSIDE PHOSPHATENORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- PubChem
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmletoposide phosphatehttps://www.ncbi.nlm.nih.gov/mesh/67061400Antineoplastic Agentshttps://www.ncbi.nlm.nih.gov/mesh/68000970
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- PATENTSCOPE (WIPO)SID 402230879https://pubchem.ncbi.nlm.nih.gov/substance/402230879
- NCBI