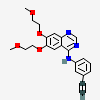
Erlotinib
- Erlotinib
- 183321-74-6
- N-(3-Ethynylphenyl)-6,7-bis(2-methoxyethoxy)quinazolin-4-amine
- Tarceva
- Erlotinib free base
- Create:2005-03-26
- Modify:2025-01-18
 Erlotinib Hydrochloride (has salt form).
Erlotinib Hydrochloride (has salt form).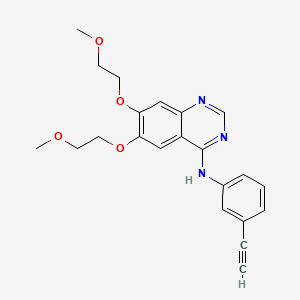
- 11C erlotinib
- 11C-erlotinib
- 358,774, CP
- 358774, CP
- CP 358,774
- CP 358774
- CP-358,774
- CP-358774
- CP358,774
- CP358774
- erlotinib
- erlotinib HCl
- erlotinib hydrochloride
- HCl, Erlotinib
- Hydrochloride, Erlotinib
- N-(3-ethynylphenyl)-6,7-bis(2-methoxyethoxy)quinazolin-4-amine
- OSI 774
- OSI-774
- OSI774
- Tarceva
- Erlotinib
- 183321-74-6
- N-(3-Ethynylphenyl)-6,7-bis(2-methoxyethoxy)quinazolin-4-amine
- Tarceva
- Erlotinib free base
- 4-Quinazolinamine, N-(3-ethynylphenyl)-6,7-bis(2-methoxyethoxy)-
- OSI-774
- 4-[(3-Ethynylphenyl)amino]-6,7-bis(2-methoxyethoxy)quinazoline
- Erlotinib [INN]
- erlotinibum
- N-(3-Ethynylphenyl)-6,7-bis(2-methoxyethoxy)-4-quinazolinamine
- UNII-J4T82NDH7E
- J4T82NDH7E
- DTXSID8046454
- Erlotinib, Free Base
- HSDB 8082
- OSI 744
- CHEBI:114785
- CP-358,774
- 183321-74-6 (free base)
- MFCD02089651
- CHEMBL553
- RG-1415
- [6,7-BIS(2-METHOXY-ETHOXY)QUINAZOLINE-4-YL]-(3-ETHYNYLPHENYL)AMINE
- CP-358774
- DTXCID6026454
- CP-35877401
- NSC 718781
- R 1415
- R-1415
- Erlotinib (INN)
- NCGC00164574-01
- [6,7-Bis-(2-methoxy-ethoxy)-quinazolin-4-yl]-(3-ethynyl-phenyl)-amine
- Erlotinib(Tarceva)
- 1429636-49-6
- CAS-183321-74-6
- Erlotinib [INN:BAN]
- SR-05000001460
- CP358774
- Erotinib
- (6,7-bis(2-methoxy-ethoxy)quinazoline-4-yl)-(3-ethynylphenyl)amine
- (6,7-Bis-(2-methoxy-ethoxy)-quinazolin-4-yl)-(3-ethynyl-phenyl)-amine
- nchembio866-comp3
- Erlotinib free base?
- Erlotinib (Standard)
- Kinome_3317
- n-(3-ethynylphenyl)-6,7-bis(2-methoxyethoxy)-4-quinazolinamine monohydrochloride
- ERLOTINIB [MI]
- ERLOTINIB [VANDF]
- ERLOTINIB [WHO-DD]
- SCHEMBL8413
- ERLOTINIB [EMA EPAR]
- BDBM5446
- cid_176870
- GTPL4920
- L01XE03
- AAKJLRGGTJKAMG-UHFFFAOYSA-N
- HMS2089F05
- HMS3244M19
- HMS3244M20
- HMS3244N19
- HMS3295A19
- HMS3713C22
- HMS3745M05
- Tox21_112202
- AC-399
- HY-50896R
- NSC800097
- s7786
- STK623143
- AKOS000282911
- Tox21_112202_1
- CCG-220420
- CS-0620
- DB00530
- NSC-800097
- Ro-508231
- SB16916
- SDCCGSBI-0634409.P005
- NCGC00164574-03
- NCGC00164574-05
- NCGC00164574-06
- NCGC00164574-14
- NCGC00164574-25
- AS-35132
- BCB03_000783
- BE164419
- HY-50896
- SY028059
- NS00006169
- D07907
- EN300-708808
- K00241
- AB01273955-01
- AB01273955-02
- AB01273955-03
- Q418369
- SR-05000001460-1
- SR-05000001460-2
- SR-05000001460-3
- SR-05000001460-6
- BRD-K70401845-001-15-7
- BRD-K70401845-003-04-7
- BRD-K70401845-003-09-6
- Z2588038919
278.0932 999
336.1351 737
304.1095 202
394.1775 196
276.0777 138
101.039 999
276.078 807
161.035 680
220.0872 654
231.0793 618
 Erlotinib Hydrochloride (has salt form)
Erlotinib Hydrochloride (has salt form)Erlotinib hydrochloride is approved to be used alone or with other drugs to treat:
• Non-small cell lung cancer (NSCLC) that is metastatic and has certain EGFR gene mutations. It may be used:
• As the first therapy.
• In patients on maintenance therapy or whose disease has gotten worse after treatment with chemotherapy.
The use of erlotinib hydrochloride to treat NSCLC that does not have the EGFR gene mutations is no longer FDA-approved.
• Pancreatic cancer. It is used with gemcitabine hydrochloride in patients whose cancer cannot be removed by surgery or has spread.
Erlotinib hydrochloride is also being studied in the treatment of other types of cancer.
- Cytoplasm
- Extracellular
- Membrane
Use (kg; approx.) in Germany (2009): >100
Use (kg; exact) in Germany (2009): 129
Consumption (g per capita; approx.) in Germany (2009): 0.00122
Consumption (g per capita; exact) in Germany (2009): 0.00158
Calculated removal (%): 12.4
Erlotinib Hydrochloride preparations (AHFS, 2012)
Table: Erlotinib Hydrochloride preparations



H302 (25%): Harmful if swallowed [Warning Acute toxicity, oral]
H351 (75%): Suspected of causing cancer [Warning Carcinogenicity]
H411 (16.7%): Toxic to aquatic life with long lasting effects [Hazardous to the aquatic environment, long-term hazard]
H413 (75%): May cause long lasting harmful effects to aquatic life [Hazardous to the aquatic environment, long-term hazard]
P203, P264, P270, P273, P280, P301+P317, P318, P330, P391, P405, and P501
(The corresponding statement to each P-code can be found at the GHS Classification page.)
Aggregated GHS information provided per 12 reports by companies from 4 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies.
Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown.
Acute Tox. 4 (25%)
Carc. 2 (75%)
Aquatic Chronic 2 (16.7%)
Aquatic Chronic 4 (75%)
Elevations in serum aminotransferase levels are common during erlotinib therapy of pancreatic and lung cancers, and values above 5 times the upper limit of normal occur in at least 10% of patients. Similar rates of ALT elevations, however, can occur with comparable antineoplastic regimens. The abnormalities are usually asymptomatic and self-limited, but may require dose adjustment or discontinuation (Case 1). In addition, there have been rare reports of clinically apparent liver injury attributed to erlotinib therapy. The time to onset is typically within days or weeks of starting therapy, and the liver injury can be severe, there being at least a dozen fatal instances reported in the literature. The onset of injury can be abrupt and the pattern of serum enzyme elevations is usually hepatocellular (Case 2). Immunoallergic features (rash, fever and eosinophilia) are not common and autoantibody formation has not been reported. Routine monitoring of liver tests during therapy is recommended. The rate of clinically significant liver injury and hepatic failure is increased in patients with preexisting cirrhosis or hepatic impairment due to liver tumor burden.
Likelihood score: B (likely but uncommon cause of clinically apparent liver injury).
M Chen, V Vijay, Q Shi, Z Liu, H Fang, W Tong. FDA-Approved Drug Labeling for the Study of Drug-Induced Liver Injury, Drug Discovery Today, 16(15-16):697-703, 2011. PMID:21624500 DOI:10.1016/j.drudis.2011.05.007
M Chen, A Suzuki, S Thakkar, K Yu, C Hu, W Tong. DILIrank: the largest reference drug list ranked by the risk for developing drug-induced liver injury in humans. Drug Discov Today 2016, 21(4): 648-653. PMID:26948801 DOI:10.1016/j.drudis.2016.02.015
◉ Summary of Use during Lactation
No information is available on the clinical use of erlotinib during breastfeeding. Because erlotinib is 93% bound to plasma proteins, the amount in milk is likely to be low. However, its half-life is about 36 hours and it might accumulate in the infant. It is also given in combination with gemcitabine for pancreatic cancer, which may increase the risk to the infant. The manufacturer recommends that breastfeeding be discontinued during erlotinib therapy and for 2 weeks after the final dose.
◉ Effects in Breastfed Infants
Relevant published information was not found as of the revision date.
◉ Effects on Lactation and Breastmilk
Relevant published information was not found as of the revision date.
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=AAKJLRGGTJKAMG-UHFFFAOYSA-N
- Exercise caution with grapefruit products. Grapefruit inhibits CYP3A4 metabolism, which may increase the serum concentration of erlotinib.
- Exercise caution with St. John's Wort. This herb induces CYP3A4 metabolism, which may reduce the serum concentration of erlotinib.
- Take on an empty stomach. Food increases erlotinib bioavailability, therefore administer at least 1 hour before or 2 hours after meals.
- BindingDBLICENSEAll data curated by BindingDB staff are provided under the Creative Commons Attribution 3.0 License (https://creativecommons.org/licenses/by/3.0/us/).https://www.bindingdb.org/rwd/bind/info.jsp
- Drug Gene Interaction database (DGIdb)LICENSEThe data used in DGIdb is all open access and where possible made available as raw data dumps in the downloads section.http://www.dgidb.org/downloads
- DrugBankLICENSECreative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode)https://www.drugbank.ca/legal/terms_of_use
- IUPHAR/BPS Guide to PHARMACOLOGYLICENSEThe Guide to PHARMACOLOGY database is licensed under the Open Data Commons Open Database License (ODbL) https://opendatacommons.org/licenses/odbl/. Its contents are licensed under a Creative Commons Attribution-ShareAlike 4.0 International License (http://creativecommons.org/licenses/by-sa/4.0/)https://www.guidetopharmacology.org/about.jsp#licenseGuide to Pharmacology Target Classificationhttps://www.guidetopharmacology.org/targets.jsp
- Therapeutic Target Database (TTD)
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/
- ChemIDplusErlotinib [INN:BAN]https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0183321746ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- EPA DSSToxCompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-notice4-[(3-Ethynylphenyl)amino]-6,7-bis(2-methoxyethoxy)quinazolinehttps://echa.europa.eu/substance-information/-/substanceinfo/100.216.0204-[(3-Ethynylphenyl)amino]-6,7-bis(2-methoxyethoxy)quinazoline (EC: 689-196-2)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/221278
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- Hazardous Substances Data Bank (HSDB)
- Human Metabolome Database (HMDB)LICENSEHMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications.http://www.hmdb.ca/citing
- ChEBI
- FDA Pharm ClassesLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingFDA Pharmacological Classificationhttps://www.fda.gov/ForIndustry/DataStandards/StructuredProductLabeling/ucm162549.htm
- LiverTox
- NCI Thesaurus (NCIt)LICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuseNCI Thesaurushttps://ncit.nci.nih.gov
- Open TargetsLICENSEDatasets generated by the Open Targets Platform are freely available for download.https://platform-docs.opentargets.org/licence
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.htmlChEMBL Protein Target Treehttps://www.ebi.ac.uk/chembl/g/#browse/targets
- ClinicalTrials.govLICENSEThe ClinicalTrials.gov data carry an international copyright outside the United States and its Territories or Possessions. Some ClinicalTrials.gov data may be subject to the copyright of third parties; you should consult these entities for any additional terms of use.https://clinicaltrials.gov/ct2/about-site/terms-conditions#Use
- Crystallography Open Database (COD)LICENSEAll data in the COD and the database itself are dedicated to the public domain and licensed under the CC0 License. Users of the data should acknowledge the original authors of the structural data.https://creativecommons.org/publicdomain/zero/1.0/
- DailyMed
- Drug Induced Liver Injury Rank (DILIrank) DatasetLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- European Medicines Agency (EMA)LICENSEInformation on the European Medicines Agency's (EMA) website is subject to a disclaimer and copyright and limited reproduction notices.https://www.ema.europa.eu/en/about-us/legal-noticeTarceva (EMEA/H/C/000618)https://www.ema.europa.eu/en/medicines/human/EPAR/tarceva
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/ERLOTINIBNORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- Drugs and Lactation Database (LactMed)
- Drugs@FDALICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- WHO Model Lists of Essential MedicinesLICENSEPermission from WHO is not required for the use of WHO materials issued under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Intergovernmental Organization (CC BY-NC-SA 3.0 IGO) license.https://www.who.int/about/policies/publishing/copyright
- EU Clinical Trials Register
- WHO Anatomical Therapeutic Chemical (ATC) ClassificationLICENSEUse of all or parts of the material requires reference to the WHO Collaborating Centre for Drug Statistics Methodology. Copying and distribution for commercial purposes is not allowed. Changing or manipulating the material is not allowed.https://www.whocc.no/copyright_disclaimer/
- National Drug Code (NDC) DirectoryLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- Japan Chemical Substance Dictionary (Nikkaji)
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.htmlAnatomical Therapeutic Chemical (ATC) classificationhttp://www.genome.jp/kegg-bin/get_htext?br08303.kegTarget-based classification of drugshttp://www.genome.jp/kegg-bin/get_htext?br08310.keg
- MassBank Europe
- Metabolomics Workbench
- Nature Chemical Biology
- NCI Cancer DrugsErlotinib Hydrochloridehttps://www.cancer.gov/about-cancer/treatment/drugs/erlotinibhydrochloride
- NIPH Clinical Trials Search of Japan
- NLM RxNorm TerminologyLICENSEThe RxNorm Terminology is created by the National Library of Medicine (NLM) and is in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from NLM. Credit to the U.S. National Library of Medicine as the source is appreciated but not required. The full RxNorm dataset requires a free license.https://www.nlm.nih.gov/research/umls/rxnorm/docs/termsofservice.html
- PharmGKBLICENSEPharmGKB data are subject to the Creative Commons Attribution-ShareALike 4.0 license (https://creativecommons.org/licenses/by-sa/4.0/).https://www.pharmgkb.org/page/policies
- PharosLICENSEData accessed from Pharos and TCRD is publicly available from the primary sources listed above. Please respect their individual licenses regarding proper use and redistribution.https://pharos.nih.gov/about
- Protein Data Bank in Europe (PDBe)
- RCSB Protein Data Bank (RCSB PDB)LICENSEData files contained in the PDB archive (ftp://ftp.wwpdb.org) are free of all copyright restrictions and made fully and freely available for both non-commercial and commercial use. Users of the data should attribute the original authors of that structural data.https://www.rcsb.org/pages/policies
- SpectraBase
- Springer Nature
- Thieme ChemistryLICENSEThe Thieme Chemistry contribution within PubChem is provided under a CC-BY-NC-ND 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc-nd/4.0/
- Wikidata
- WikipediaTobramycinhttps://en.wikipedia.org/wiki/Tobramycin
- Wiley
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlErlotinib Hydrochloridehttps://www.ncbi.nlm.nih.gov/mesh/2009966Antineoplastic Agentshttps://www.ncbi.nlm.nih.gov/mesh/68000970Tyrosine Kinase Inhibitorshttps://www.ncbi.nlm.nih.gov/mesh/2103139
- PubChem
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 388492510https://pubchem.ncbi.nlm.nih.gov/substance/388492510
- NCBI