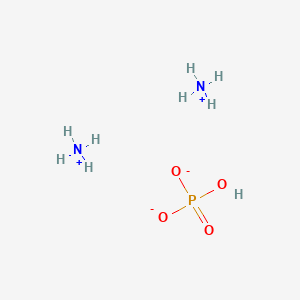
Ammonium Phosphate, Dibasic
- (NH4)2HPO4
- H9N2O4P
- 7783-28-0
- Diammonium hydrogenphosphate
- Diammonium hydrogen phosphate
- DIAMMONIUM PHOSPHATE
- Ammonium phosphate dibasic
- Create:2005-08-08
- Modify:2025-02-01
7722-76-1
7783-28-0
- ammonium hydrogen phosphate
- ammonium orthophosphate
- ammonium phosphate
- ammonium phosphate ((NH4)3PO4)
- ammonium phosphate, dibasic
- ammonium phosphate, monobasic
- ammonium phosphate, tribasic
- diammonium hydrogen phosphate
- diammonium phosphate
- monoammonium phosphate
- monobasic ammonium phosphate
- phosphoric acid, ammonium salt
- primary ammonum phosphate
- triammonium phosphate
- tribasic ammonium phosphate
- 7783-28-0
- Diammonium hydrogenphosphate
- Diammonium hydrogen phosphate
- DIAMMONIUM PHOSPHATE
- Ammonium phosphate dibasic
- Phosphoric acid, diammonium salt
- Akoustan A
- Fyrex
- Pelor
- Dibasic ammonium phosphate
- Ammonium phosphate, dibasic
- Coaltrol LPA 445
- Diammonium orthophosphate
- K2 (phosphate)
- Diammonium hydrogen orthophosphate
- Secondary ammonium phosphate
- Phos-Chek 202A
- Phos-Chek 259
- Hydrogen diammonium phosphate
- Caswell No. 286C
- Ammonium orthophosphate dibasic
- Diammonium acid phosphate
- Diammonium monohydrogen phosphate
- Ammonium monohydrogen orthophosphate
- Ammonium phosphate, secondary
- HSDB 301
- Diammonium hydrogenorthophosphate
- Pyrozyl war
- UNII-10LGE70FSU
- diazanium hydrogen phosphate
- diazanium;hydrogen phosphate
- EINECS 231-987-8
- 10LGE70FSU
- ammonium monohydrogen phosphate
- Ammonium phosphate ((NH4)2(HPO4))
- Ammonium phosphoricum
- Spartan ar 295
- (NH4)2HPO4
- AI3-25349
- Ammoniumhydrogenphosphate
- Ammonium hydrogenphosphate
- Ammonium phosphate [NF]
- Ammonium phosphate,dibasic
- DTXSID6029705
- CHEBI:63051
- EC 231-987-8
- Phosphoric acid, ammonium salt (1:2)
- Ammonium phosphate (NF)
- AMMONIUM PHOSPHATE (MART.)
- AMMONIUM PHOSPHATE [MART.]
- AMMONIUM PHOSPHATE DIBASIC (USP-RS)
- AMMONIUM PHOSPHATE DIBASIC [USP-RS]
- 287488-13-5
- PHOS-CHEK 202
- di-ammonium phosphate
- Ammonium phosphatedi basic
- Ammonium phosphate secondary
- DTXCID109689
- DTXCID309705
- AMMONIUM PHOSPHATE [HSDB]
- AMMONIUM PHOSPHATE [VANDF]
- AMMONIUM PHOSPHORICUM [HPUS]
- AKOS015856700
- AMMONIUM PHOSPHATE, DIBASIC [II]
- AMMONIUM PHOSPHATE, DIBASIC [MI]
- AMMONIUM PHOSPHATE, DIBASIC [FCC]
- DA-62836
- AMMONIUM PHOSPHATE,DIBASIC [WHO-DD]
- NS00093532
- D02921
Phosphate Ion (has active moiety)
- Ammonium phosphate, dibasic; baptisia tinctoria root; cinchona officinalis bark; entamoeba histolytica; garlic; wormwood (component of)
- Activated charcoal; ammonium phosphate, dibasic; amoeba proteus; arsenic trioxide; cinchona officinalis bark; garlic; veratrum album root; wormwood (component of)
- Ammonium phosphate, dibasic; atropa belladonna; colchicum autumnale bulb; formic acid; fraxinus excelsior leaf; rhododendron tomentosum leafy twig; sodium carbonate; strychnos nux-vomica seed; urtica urens whole (component of)
Soldering [Category: Heat or Machine]
Pulp and Paper Processing [Category: Industry]
Textiles (Printing, Dyeing, or Finishing) [Category: Industry]
Farming (Feed Additives) [Category: Industry]
- Flame retardants
- Processing aids, not otherwise listed
- Brightener
- Agricultural chemicals (non-pesticidal)
- Soil amendments (fertilizers)
- Other
- Flame retardant
- Not Known or Reasonably Ascertainable
- Adhesion/cohesion promoter
- Agricultural chemicals (non-pesticidal)
- Brightener
- Flame retardants
- Other
- Soil amendments (fertilizers)
- Not Known or Reasonably Ascertainable
Information on 66 consumer products that contain Ammonium phosphate, dibasic in the following categories is provided:
• Inside the Home
• Landscaping/Yard
• Pesticides
2019: 5,000,000,000 - <10,000,000,000 lb
2018: 5,000,000,000 - <10,000,000,000 lb
2017: 5,000,000,000 - <10,000,000,000 lb
2016: 5,000,000,000 - <10,000,000,000 lb
- All Other Chemical Product and Preparation Manufacturing
- Not Known or Reasonably Ascertainable
- Fabricated Metal Product Manufacturing
- Wood Product Manufacturing
- Computer and Electronic Product Manufacturing
- All Other Basic Inorganic Chemical Manufacturing
- Pesticide, Fertilizer, and Other Agricultural Chemical Manufacturing
- Transportation Equipment Manufacturing
- Machinery Manufacturing
- Agriculture, Forestry, Fishing and Hunting
- Electrical Equipment, Appliance, and Component Manufacturing
- Food, beverage, and tobacco product manufacturing
- Wholesale and Retail Trade

H315 (18.1%): Causes skin irritation [Warning Skin corrosion/irritation]
H319 (18.2%): Causes serious eye irritation [Warning Serious eye damage/eye irritation]
H335 (15.2%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation]
P261, P264, P264+P265, P271, P280, P302+P352, P304+P340, P305+P351+P338, P319, P321, P332+P317, P337+P317, P362+P364, P403+P233, P405, and P501
(The corresponding statement to each P-code can be found at the GHS Classification page.)
Aggregated GHS information provided per 658 reports by companies from 23 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies.
Reported as not meeting GHS hazard criteria per 530 of 658 reports by companies. For more detailed information, please visit ECHA C&L website.
There are 20 notifications provided by 128 of 658 reports by companies with hazard statement code(s).
Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown.
Skin Irrit. 2 (18.1%)
Eye Irrit. 2 (18.2%)
STOT SE 3 (15.2%)
INHALATION: if exposed to ammonia fumes from diammonium phosphate, give artificial respiration and oxygen if needed; enforce rest.
EYES: flush with water for at least 15 min.; if irritation persists, get medical attention.
SKIN: flush with water. (USCG, 1999)
IMAP assessments - Phosphoric acid, diammonium salt: Human health tier I assessment
IMAP assessments - Phosphoric acid, diammonium salt: Environment tier I assessment
Skin Sensitizer - An agent that can induce an allergic reaction in the skin.
Toxic Pneumonitis - Inflammation of the lungs induced by inhalation of metal fumes or toxic gases and vapors.
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=MNNHAPBLZZVQHP-UHFFFAOYSA-N
- Australian Industrial Chemicals Introduction Scheme (AICIS)Phosphoric acid, diammonium salthttps://services.industrialchemicals.gov.au/search-assessments/Phosphoric acid, diammonium salthttps://services.industrialchemicals.gov.au/search-inventory/
- CAMEO ChemicalsLICENSECAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data.https://cameochemicals.noaa.gov/help/reference/terms_and_conditions.htm?d_f=falseAMMONIUM PHOSPHATEhttps://cameochemicals.noaa.gov/chemical/8261CAMEO Chemical Reactivity Classificationhttps://cameochemicals.noaa.gov/browse/react
- ChemIDplusAmmonium phosphate [NF]https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0007783280ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- EPA Chemical Data Reporting (CDR)LICENSEThe U.S. Government retains a nonexclusive, royalty-free license to publish or reproduce these documents, or allow others to do so, for U.S. Government purposes. These documents may be freely distributed and used for non-commercial, scientific and educational purposes.https://www.epa.gov/web-policies-and-procedures/epa-disclaimers#copyrightPhosphoric acid, ammonium salt (1:2)https://www.epa.gov/chemical-data-reporting
- EPA Chemicals under the TSCAPhosphoric acid, ammonium salt (1:2)https://www.epa.gov/chemicals-under-tscaEPA TSCA Classificationhttps://www.epa.gov/tsca-inventory
- EPA DSSToxDiammonium hydrogen phosphatehttps://comptox.epa.gov/dashboard/DTXSID6029705CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- EPA Provisional Peer-Reviewed Toxicity Values (PPRTVs)Diammonium phosphatehttps://cfpub.epa.gov/ncea/pprtv/chemicalLanding.cfm?pprtv_sub_id=1668
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-noticeDiammonium hydrogenorthophosphatehttps://chem.echa.europa.eu/100.029.079Diammonium hydrogenorthophosphate (EC: 231-987-8)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/47307
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingAMMONIUM PHOSPHATE, DIBASIChttps://gsrs.ncats.nih.gov/ginas/app/beta/substances/10LGE70FSU
- Hazardous Substances Data Bank (HSDB)DIAMMONIUM HYDROGEN PHOSPHATEhttps://pubchem.ncbi.nlm.nih.gov/source/hsdb/301
- ILO-WHO International Chemical Safety Cards (ICSCs)AMMONIUM PHOSPHATE DIBASIChttps://www.ilo.org/dyn/icsc/showcard.display?p_version=2&p_card_id=0217
- New Zealand Environmental Protection Authority (EPA)LICENSEThis work is licensed under the Creative Commons Attribution-ShareAlike 4.0 International licence.https://www.epa.govt.nz/about-this-site/general-copyright-statement/Ammonium monohydrogen orthophosphatehttps://www.epa.govt.nz/industry-areas/hazardous-substances/guidance-for-importers-and-manufacturers/hazardous-substances-databases/
- Risk Assessment Information System (RAIS)LICENSEThis work has been sponsored by the U.S. Department of Energy (DOE), Office of Environmental Management, Oak Ridge Operations (ORO) Office through a joint collaboration between United Cleanup Oak Ridge LLC (UCOR), Oak Ridge National Laboratory (ORNL), and The University of Tennessee, Ecology and Evolutionary Biology, The Institute for Environmental Modeling (TIEM). All rights reserved.https://rais.ornl.gov/Diammonium phosphatehttps://rais.ornl.gov/cgi-bin/tools/TOX_search
- EU Pesticides Database
- Haz-Map, Information on Hazardous Chemicals and Occupational DiseasesLICENSECopyright (c) 2022 Haz-Map(R). All rights reserved. Unless otherwise indicated, all materials from Haz-Map are copyrighted by Haz-Map(R). No part of these materials, either text or image may be used for any purpose other than for personal use. Therefore, reproduction, modification, storage in a retrieval system or retransmission, in any form or by any means, electronic, mechanical or otherwise, for reasons other than personal use, is strictly prohibited without prior written permission.https://haz-map.com/AboutAmmonium phosphate, dibasichttps://haz-map.com/Agents/7963
- ChEBIDiammonium hydrogen phosphatehttps://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:63051
- Consumer Product Information Database (CPID)LICENSECopyright (c) 2024 DeLima Associates. All rights reserved. Unless otherwise indicated, all materials from CPID are copyrighted by DeLima Associates. No part of these materials, either text or image may be used for any purpose other than for personal use. Therefore, reproduction, modification, storage in a retrieval system or retransmission, in any form or by any means, electronic, mechanical or otherwise, for reasons other than personal use, is strictly prohibited without prior written permission.https://www.whatsinproducts.com/contents/view/1/6Ammonium phosphate, dibasichttps://www.whatsinproducts.com/chemicals/view/1/2112/007783-28-0Consumer Products Category Classificationhttps://www.whatsinproducts.com/
- Cosmetic Ingredient Review (CIR)
- EPA Chemical and Products Database (CPDat)Diammonium hydrogen phosphatehttps://comptox.epa.gov/dashboard/DTXSID6029705#exposureEPA CPDat Classificationhttps://www.epa.gov/chemical-research/chemical-and-products-database-cpdat
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/NORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- DailyMedAMMONIUM PHOSPHATE, DIBASIChttps://dailymed.nlm.nih.gov/dailymed/search.cfm?labeltype=all&query=AMMONIUM+PHOSPHATE,+DIBASIC
- FDA Substances Added to FoodLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingAMMONIUM PHOSPHATE, DIBASIChttps://www.hfpappexternal.fda.gov/scripts/fdcc/index.cfm?set=FoodSubstances&id=AMMONIUMPHOSPHATEDIBASIC
- Joint FAO/WHO Expert Committee on Food Additives (JECFA)LICENSEPermission from WHO is not required for the use of WHO materials issued under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Intergovernmental Organization (CC BY-NC-SA 3.0 IGO) licence.https://www.who.int/about/policies/publishing/copyrightDIAMMONIUM HYDROGEN PHOSPHATEhttps://apps.who.int/food-additives-contaminants-jecfa-database/Home/Chemical/569
- USGS Columbia Environmental Research CenterLICENSEhttps://www.usgs.gov/foia
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.html
- National Drug Code (NDC) DirectoryLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingAMMONIUM PHOSPHATE, DIBASIChttps://www.fda.gov/drugs/drug-approvals-and-databases/national-drug-code-directory
- NCI Thesaurus (NCIt)LICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuseNCI Thesaurushttps://ncit.nci.nih.gov
- NLM RxNorm TerminologyLICENSEThe RxNorm Terminology is created by the National Library of Medicine (NLM) and is in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from NLM. Credit to the U.S. National Library of Medicine as the source is appreciated but not required. The full RxNorm dataset requires a free license.https://www.nlm.nih.gov/research/umls/rxnorm/docs/termsofservice.htmlammonium phosphate, dibasichttps://rxnav.nlm.nih.gov/id/rxnorm/1427061
- SpectraBaseDIAMMONIUM HYDROGEN PHOSPHATE (sec)https://spectrabase.com/spectrum/7nrF3CZonAYDIAMMONIUM HYDROGEN PHOSPHATE (sec)https://spectrabase.com/spectrum/4eZy9LwT3s2DIAMMONIUM PHOSPHATEhttps://spectrabase.com/spectrum/EJV0aXBYtS6AMMONIUM PHOSPHATE, DIBASIChttps://spectrabase.com/spectrum/GRsZf8Rycqd
- Springer Nature
- Thieme ChemistryLICENSEThe Thieme Chemistry contribution within PubChem is provided under a CC-BY-NC-ND 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc-nd/4.0/
- Wikidatadibasic ammonium phosphatehttps://www.wikidata.org/wiki/Q418889
- Wikipediaammonium phosphatehttps://en.wikipedia.org/wiki/Ammonium_phosphateDiammonium phosphatehttps://en.wikipedia.org/wiki/Diammonium_phosphate
- Wiley
- PubChem
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlammonium phosphatehttps://www.ncbi.nlm.nih.gov/mesh/67024788Food Additiveshttps://www.ncbi.nlm.nih.gov/mesh/68005503Fertilizershttps://www.ncbi.nlm.nih.gov/mesh/68005308
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- EPA Substance Registry ServicesEPA SRS List Classificationhttps://sor.epa.gov/sor_internet/registry/substreg/LandingPage.do
- PATENTSCOPE (WIPO)SID 403029563https://pubchem.ncbi.nlm.nih.gov/substance/403029563

 CID 1004 (Phosphoric Acid)
CID 1004 (Phosphoric Acid) CID 222 (Ammonia)
CID 222 (Ammonia)