Dehydroacetic acid (sodium salt)
- SODIUM DEHYDROACETATE
- Dehydroacetic acid sodium salt
- Dehydroacetic acid (sodium salt)
- 2-Acetyl-5-hydroxy-3-oxo-4-hexenoic acid delta-lactone sodium salt
- SCHEMBL41960
- Create:2008-02-05
- Modify:2025-01-11
 Sodium Dehydroacetate (annotation moved to).
Sodium Dehydroacetate (annotation moved to).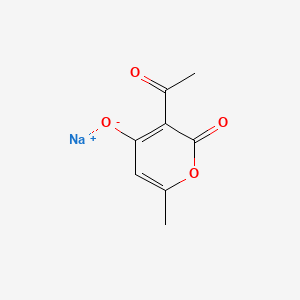
- dehydroacetic acid
- dehydroacetic acid ion (1-)
- dehydroacetic acid, potassium ion (1-)
- dehydroacetic acid, sodium ion (1-)
- dehydroacetic acid, sodium monohydrate ion (1-)
- dehydroacetic acid, zinc ion (1-)
- DHA-S
- DHAS
- dihydroxyacetone sulfate
- sodium dehydroacetate
- SODIUM DEHYDROACETATE
- Dehydroacetic acid sodium salt
- Dehydroacetic acid (sodium salt)
- 2-Acetyl-5-hydroxy-3-oxo-4-hexenoic acid delta-lactone sodium salt
- SCHEMBL41960
- CHEMBL2107528
- ZPNRBQVNNIDJHX-UHFFFAOYSA-M
- MFCD00040583
- AKOS037643347
- Dehydroacetic acid, sodium salt; >98%
- AS-14861
- D0040
- sodium 3-acetyl-6-methyl-2-oxo-2H-pyran-4-olate
- 3-Acetyl-4-hydroxy-6-methyl-2-pyrone Sodium Salt
- SODIUM 3-ACETYL-6-METHYL-2-OXOPYRAN-4-OLATE
- Sodium 3-Acetyl-4-hydroxy-6-methyl-2-oxo-4(2H)-pyranolate
 Sodium Dehydroacetate (annotation moved to)
Sodium Dehydroacetate (annotation moved to)Information on 78 consumer products that contain Sodium dehydroacetate in the following categories is provided:
• Personal Care

P264, P270, P301+P317, P330, and P501
(The corresponding statement to each P-code can be found at the GHS Classification page.)
EYES: First check the victim for contact lenses and remove if present. Flush victim's eyes with water or normal saline solution for 20 to 30 minutes while simultaneously calling a hospital or poison control center. Do not put any ointments, oils, or medication in the victim's eyes without specific instructions from a physician. IMMEDIATELY transport the victim after flushing eyes to a hospital even if no symptoms (such as redness or irritation) develop.
SKIN: IMMEDIATELY flood affected skin with water while removing and isolating all contaminated clothing. Gently wash all affected skin areas thoroughly with soap and water. If symptoms such as redness or irritation develop, IMMEDIATELY call a physician and be prepared to transport the victim to a hospital for treatment.
INHALATION: IMMEDIATELY leave the contaminated area; take deep breaths of fresh air. If symptoms (such as wheezing, coughing, shortness of breath, or burning in the mouth, throat, or chest) develop, call a physician and be prepared to transport the victim to a hospital. Provide proper respiratory protection to rescuers entering an unknown atmosphere. Whenever possible, Self-Contained Breathing Apparatus (SCBA) should be used; if not available, use a level of protection greater than or equal to that advised under Protective Clothing.
INGESTION: DO NOT INDUCE VOMITING. If the victim is conscious and not convulsing, give 1 or 2 glasses of water to dilute the chemical and IMMEDIATELY call a hospital or poison control center. Be prepared to transport the victim to a hospital if advised by a physician. If the victim is convulsing or unconscious, do not give anything by mouth, ensure that the victim's airway is open and lay the victim on his/her side with the head lower than the body. DO NOT INDUCE VOMITING. IMMEDIATELY transport the victim to a hospital. (NTP, 1992)
SMALL SPILLS AND LEAKAGE: If you spill this chemical, you should dampen the solid spill material with water, then transfer the dampened material to a suitable container. Use absorbent paper dampened with water to pick up any remaining material. Seal your contaminated clothing and the absorbent paper in a vapor-tight plastic bag for eventual disposal. Wash all contaminated surfaces with a soap and water solution. Do not reenter the contaminated area until the Safety Officer (or other responsible person) has verified that the area has been properly cleaned.
STORAGE PRECAUTIONS: You should store this material in a refrigerator. (NTP, 1992)
Esters, Sulfate Esters, Phosphate Esters, Thiophosphate Esters, and Borate Esters
Phenolic Salts
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=ZPNRBQVNNIDJHX-UHFFFAOYSA-M
- CAMEO ChemicalsLICENSECAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data.https://cameochemicals.noaa.gov/help/reference/terms_and_conditions.htm?d_f=falseSODIUM DEHYDROACETATEhttps://cameochemicals.noaa.gov/chemical/21018CAMEO Chemical Reactivity Classificationhttps://cameochemicals.noaa.gov/browse/react
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingSODIUM DEHYDROACETATEhttps://gsrs.ncats.nih.gov/ginas/app/beta/substances/8W46YN971G
- Hazardous Substances Data Bank (HSDB)SODIUM DEHYDROACETATEhttps://pubchem.ncbi.nlm.nih.gov/source/hsdb/4236
- New Zealand Environmental Protection Authority (EPA)LICENSEThis work is licensed under the Creative Commons Attribution-ShareAlike 4.0 International licence.https://www.epa.govt.nz/about-this-site/general-copyright-statement/
- Haz-Map, Information on Hazardous Chemicals and Occupational DiseasesLICENSECopyright (c) 2022 Haz-Map(R). All rights reserved. Unless otherwise indicated, all materials from Haz-Map are copyrighted by Haz-Map(R). No part of these materials, either text or image may be used for any purpose other than for personal use. Therefore, reproduction, modification, storage in a retrieval system or retransmission, in any form or by any means, electronic, mechanical or otherwise, for reasons other than personal use, is strictly prohibited without prior written permission.https://haz-map.com/AboutSodium dehydroacetatehttps://haz-map.com/Agents/7096
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.html
- Consumer Product Information Database (CPID)LICENSECopyright (c) 2024 DeLima Associates. All rights reserved. Unless otherwise indicated, all materials from CPID are copyrighted by DeLima Associates. No part of these materials, either text or image may be used for any purpose other than for personal use. Therefore, reproduction, modification, storage in a retrieval system or retransmission, in any form or by any means, electronic, mechanical or otherwise, for reasons other than personal use, is strictly prohibited without prior written permission.https://www.whatsinproducts.com/contents/view/1/6Sodium dehydroacetatehttps://www.whatsinproducts.com/chemicals/view/1/1050/004418-26-2Consumer Products Category Classificationhttps://www.whatsinproducts.com/
- Cosmetic Ingredient Review (CIR)
- FDA Substances Added to FoodLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- Japan Chemical Substance Dictionary (Nikkaji)
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.html
- NITE-CMCSodium dehydroacetate - FY2012 (New/original classication)https://www.chem-info.nite.go.jp/chem/english/ghs/12-mhlw-0019e.html
- NLM RxNorm TerminologyLICENSEThe RxNorm Terminology is created by the National Library of Medicine (NLM) and is in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from NLM. Credit to the U.S. National Library of Medicine as the source is appreciated but not required. The full RxNorm dataset requires a free license.https://www.nlm.nih.gov/research/umls/rxnorm/docs/termsofservice.htmlsodium dehydroacetatehttps://rxnav.nlm.nih.gov/id/rxnorm/1366847
- SpectraBase3-ACETYL-4-HYDROXY-6-METHYL-2H-PYRAN-2-ONE, SODIUM SALThttps://spectrabase.com/spectrum/BXZNCmUyBhY
- Springer Nature
- WikipediaSodium dehydroacetatehttps://en.wikipedia.org/wiki/Sodium_dehydroacetate
- PubChem
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmldehydroacetic acidhttps://www.ncbi.nlm.nih.gov/mesh/67027493
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/NORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 403447395https://pubchem.ncbi.nlm.nih.gov/substance/403447395
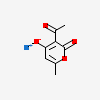

 CID 54678494 (3-Acetyl-4-hydroxy-6-methyl-2H-pyran-2-one)
CID 54678494 (3-Acetyl-4-hydroxy-6-methyl-2H-pyran-2-one) CID 5360545 (Sodium)
CID 5360545 (Sodium) CID 10623
CID 10623