Leucovorin Calcium
- calcium folinate
- LEUCOVORIN CALCIUM
- 1492-18-8
- Folinic acid calcium salt
- Calcium leucovorin
- Create:2019-01-15
- Modify:2025-01-18
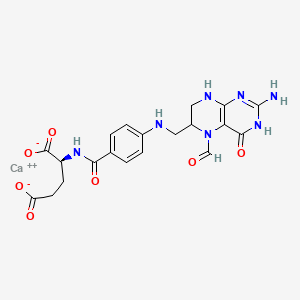
- 5 Formyltetrahydrofolate
- 5 Formyltetrahydropteroylglutamate
- 5-Formyltetrahydrofolate
- 5-Formyltetrahydropteroylglutamate
- Acid, Folinic
- Calcium Folinate
- Calcium Leucovorin
- Citrovorum Factor
- Factor, Citrovorum
- Folinate, Calcium
- Folinic Acid
- Folinic Acid SF
- Folinic Acid-SF
- Leucovorin
- Leucovorin, (D)-Isomer
- Leucovorin, (DL)-Isomer
- Leucovorin, (R)-Isomer
- Leucovorin, Calcium
- Leucovorin, Calcium (1:1) Salt
- Leucovorin, Calcium (1:1) Salt, (DL)-Isomer
- Leucovorin, Calcium (1:1) Salt, Pentahydrate
- Leucovorin, Monosodium Salt
- Leukovorin
- Leukovorum
- Monosodium Salt Leucovorin
- N(5)-Formyltetrahydrofolate
- Wellcovorin
- calcium folinate
- LEUCOVORIN CALCIUM
- 1492-18-8
- Folinic acid calcium salt
- Calcium leucovorin
- Lederfoline
- Rescuvolin
- Wellcovorin
- Folinate-SF Calcium
- Folidan
- Calcii folinas
- Folinato calcico
- Leucovorin calcium salt
- Leucovorin calcium preservative free
- Calcium citrovorum factor
- Calcium L-folinate
- Folinate de calcium
- Folinic acid-SF, calcium salt
- Lederfolat
- Lederfolin
- Folaren
- Foliben
- Leucosar
- Tonofolin
- Folinic acid (Calcium)
- Calcium 5-formyltetrahydrofolate
- folinate calcium
- Anhydrous calcium folinate
- UNII-RPR1R4C0P4
- (+)-L-Folinic acid, calcium salt
- NSC 3590
- EINECS 216-082-8
- RPR1R4C0P4
- Calcii folinas [INN-Latin]
- Folinato calcico [INN-Spanish]
- Folinate de calcium [INN-French]
- Calcium folinate [INN]
- NSC-3590
- Leucovorin calcium [USP]
- LEVOLEUCOVORIN CALCIUM
- Folinic acid calcium salt hydrate
- Calcium N-(p-((((6RS)-2-amino-5-formyl-5,6,7,8-tetrahydro-4-hydroxy-6-pteridinyl)methyl)amino)benzoyl)-L-glutamate (1:1)
- calciumfolinate
- Rescufolin
- Fusilev
- 80433-71-2
- L-Glutamic acid, N-(4-(((2-amino-5-formyl-1,4,5,6,7,8-hexahydro-4-oxo-6-pteridinyl)methyl)amino)benzoyl)-, calcium salt (1:1)
- L-Glutamic acid, N-[4-[[(2-amino-5-formyl-1,4,5,6,7,8-hexahydro-4-oxo-6-pteridinyl)methyl]amino]benzoyl]-, calcium salt (1:1)
- FOLINIC ACID (AS CALCIUM FOLINATE)
- Leucovorin calcium (USP)
- Calcii folinas (INN-Latin)
- Glutamic acid, N-(p-(((2-amino-5-formyl-5,6,7,8-tetrahydro-3-hydroxy-6-pteridinyl)methyl)amino)benzoyl)-, calcium salt (1:1), L-
- Folinato calcico (INN-Spanish)
- CALCIUM FOLINATE (MART.)
- CALCIUM FOLINATE [MART.]
- Folinate de calcium (INN-French)
- Calcium folinate pentahydrate
- LEUCOVORIN CALCIUM (USP-RS)
- LEUCOVORIN CALCIUM [USP-RS]
- CALCIUM FOLINATE (EP IMPURITY)
- CALCIUM FOLINATE [EP IMPURITY]
- NSC3590
- Calcium (4-(((2-amino-5-formyl-4-oxo-1,4,5,6,7,8-hexahydropteridin-6-yl)methyl)amino)benzoyl)-L-glutamate
- calcium;(2S)-2-[[4-[(2-amino-5-formyl-4-oxo-3,6,7,8-tetrahydropteridin-6-yl)methylamino]benzoyl]amino]pentanedioate
- LEUCOVORIN CALCIUM (USP MONOGRAPH)
- LEUCOVORIN CALCIUM [USP MONOGRAPH]
- Calcifolin
- Adinepar
- Calfolex
- Calinat
- Cehafolin
- Citofolin
- Dalisol
- Disintox
- Divical
- Folacal
- Folaxin
- Folidar
- Folinac
- Folinoral
- Folinvit
- Foliplus
- Citrec
- Ecofol
- Emovis
- Folix
- Uzel
- Calc Folinate
- Leucovorin Ca
- Flynoken A
- FOLI-cell
- 5-Formyl-5,6,7,8-tetrahydrofolic acid calcium salt hydrate
- Prestwick_224
- Cromatonbic Folinico
- 5-formyl tetrahydrofolate
- SCHEMBL18573
- SPECTRUM1500364
- 5-Formyl-5,6,7,8-tetrahydrofolic acid calcium salt
- CALCIUM FOLINATE [JAN]
- CALCIUM FOLINATE [VANDF]
- CHEMBL1201138
- CHEBI:31340
- HMS500L04
- DTXSID20872448
- CALCIUM FOLINATE [WHO-DD]
- Folfox component leucovorin calcium
- HMS1570C18
- HMS1920J05
- HMS2091P21
- HMS2097C18
- HMS3714C18
- LEUCOVORIN CALCIUM [VANDF]
- Folinic acid (calcium) (Standard)
- BDBM50247893
- CCG-38930
- HY-13664R
- AKOS015894859
- AKOS032949578
- AC-7456
- CCG-269782
- CS-1363
- LEUCOVORIN CALCIUM [ORANGE BOOK]
- AS-13746
- BC164621
- Folinate calcium 100 microg/mL in Water
- HY-13664
- C2235
- NS00127423
- F-7000
- EN300-6735051
- Q27077063
- Z2683233771
- calcium (2S)-2-[(4-{[(2-amino-5-formyl-4-oxo-1,4,5,6,7,8-hexahydropteridin-6-yl)methyl]amino}phenyl)formamido]pentanedioate
- N-(4-(((2-Amino-5-formyl-1,4,5,6,7,8-hexahydro-4-oxo-6-pteridinyl)methyl)amnio)benzoyl)-L-glutamic Acid, Calcium Salt (1:1)
- N-(p-(((2-Amino-5-formyl-5,6,7,8-tetrahydro-4-hydroxy-6-pteridinyl)methyl)amino)benzoyl)glutamic Acid
202.35 Ų [M-H]- [CCS Type: DT; Method: stepped-field]
198.8 Ų [M+H]+ [CCS Type: DT; Method: stepped-field]
Leucovorin (has active moiety)
- Leucovorin calcium; pyrimethamine (component of)
Leucovorin calcium is approved to be used alone or with other drugs to treat:
• Colorectal cancer. It is used with fluorouracil as palliative treatment in patients with advanced disease.
• Anemia. It is used to treat megaloblastic anemia that occurs when the body does not get enough of a vitamin called folic acid. It is used by patients who cannot take the vitamin by mouth.
Leucovorin calcium is also used to prevent and treat the toxic effects of high-dosemethotrexate when used to treat osteosarcoma and other types of cancer. It is also used to treat overdoses of methotrexate or other folic acid antagonists. The drug is also being studied in the treatment of other conditions and types of cancer.
Use (kg; approx.) in Germany (2009): >250
Consumption (g per capita; approx.) in Germany (2009): 0.00305
Calculated removal (%): 22


H315 (94.2%): Causes skin irritation [Warning Skin corrosion/irritation]
H317 (88.5%): May cause an allergic skin reaction [Warning Sensitization, Skin]
H319 (94.2%): Causes serious eye irritation [Warning Serious eye damage/eye irritation]
H334 (86.5%): May cause allergy or asthma symptoms or breathing difficulties if inhaled [Danger Sensitization, respiratory]
H335 (92.3%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation]
P233, P260, P261, P264, P264+P265, P271, P272, P280, P284, P302+P352, P304+P340, P305+P351+P338, P319, P321, P332+P317, P333+P317, P337+P317, P342+P316, P362+P364, P403, P403+P233, P405, and P501
(The corresponding statement to each P-code can be found at the GHS Classification page.)
Aggregated GHS information provided per 52 reports by companies from 11 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies.
Reported as not meeting GHS hazard criteria per 2 of 52 reports by companies. For more detailed information, please visit ECHA C&L website.
There are 9 notifications provided by 50 of 52 reports by companies with hazard statement code(s).
Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown.
Skin Irrit. 2 (94.2%)
Skin Sens. 1 (88.5%)
Eye Irrit. 2 (94.2%)
Resp. Sens. 1 (86.5%)
STOT SE 3 (92.3%)
Skin Irrit. 2 (100%)
Skin Sens. 1 (100%)
Eye Irrit. 2 (97.5%)
Resp. Sens. 1 (97.5%)
STOT SE 3 (97.5%)
◉ Summary of Use during Lactation
Leucovorin (folinic acid; 5-formyltetrahydrofolic acid) and its levo- isomer, levoleucovorin, are folic acid derivatives that are normal components of breastmilk. Because leucovorin and levoleucovorin are used as therapeutic agents with potentially toxic drugs such as fluorouracil or methotrexate, the LactMed record of the drug it is used with should be consulted.
◉ Effects in Breastfed Infants
Relevant published information was not found as of the revision date.
◉ Effects on Lactation and Breastmilk
Relevant published information was not found as of the revision date.
- Australian Industrial Chemicals Introduction Scheme (AICIS)L-Glutamic acid, N-[4-[[(2-amino-5-formyl-1,4,5,6,7,8-hexahydro-4-oxo-6-pteridinyl)methyl]amino]benzoyl]-, calcium salt(1:1)https://services.industrialchemicals.gov.au/search-inventory/
- ChemIDplusLeucovorin calcium [USP]https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0001492188ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- EPA DSSToxFolinic acid calcium salthttps://comptox.epa.gov/dashboard/DTXSID20872448CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-noticeFolinic acid calcium salt hydratehttps://echa.europa.eu/substance-information/-/substanceinfo/100.151.674N-[4-[[(2-Amino-5-formyl-1,4,5,6,7,8- hexahydro-4-oxo-6-pteridinyl)-methyl]amino]benzoyl], L-glutamic Acid Calcium Salt pentahydratehttps://echa.europa.eu/substance-information/-/substanceinfo/100.228.231Calcium folinate (EC: 216-082-8)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/75462Folinic acid calcium salt hydrate (EC: 622-950-0)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/156273N-[4-[[(2-Amino-5-formyl-1,4,5,6,7,8- hexahydro-4-oxo-6-pteridinyl)-methyl]amino]benzoyl], L-glutamic Acid Calcium Salt pentahydrate (EC: 801-530-2)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/234758
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingLEUCOVORIN CALCIUMhttps://gsrs.ncats.nih.gov/ginas/app/beta/substances/RPR1R4C0P4
- New Zealand Environmental Protection Authority (EPA)LICENSEThis work is licensed under the Creative Commons Attribution-ShareAlike 4.0 International licence.https://www.epa.govt.nz/about-this-site/general-copyright-statement/
- CCSbaseCCSbase Classificationhttps://ccsbase.net/
- ChEBICalcium folinatehttps://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:31340
- NCI Thesaurus (NCIt)LICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuseNCI Thesaurushttps://ncit.nci.nih.gov
- Open TargetsLICENSEDatasets generated by the Open Targets Platform are freely available for download.https://platform-docs.opentargets.org/licenceLEUCOVORIN CALCIUMhttps://platform.opentargets.org/drug/CHEMBL1201138
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.htmlChEMBL Protein Target Treehttps://www.ebi.ac.uk/chembl/g/#browse/targets
- ClinicalTrials.govLICENSEThe ClinicalTrials.gov data carry an international copyright outside the United States and its Territories or Possessions. Some ClinicalTrials.gov data may be subject to the copyright of third parties; you should consult these entities for any additional terms of use.https://clinicaltrials.gov/ct2/about-site/terms-conditions#Use
- DailyMed
- Drug Gene Interaction database (DGIdb)LICENSEThe data used in DGIdb is all open access and where possible made available as raw data dumps in the downloads section.http://www.dgidb.org/downloadsLEUCOVORIN CALCIUMhttps://www.dgidb.org/drugs/rxcui:225852
- Therapeutic Target Database (TTD)Leucovorin Calciumhttps://idrblab.net/ttd/data/drug/details/D0B6TW
- Drugs and Lactation Database (LactMed)
- Drugs@FDALICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingLEUCOVORIN CALCIUMhttps://www.accessdata.fda.gov/scripts/cder/daf/
- EU Clinical Trials Register
- FDA Orange BookLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.html
- National Drug Code (NDC) DirectoryLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- NCI Cancer DrugsLeucovorin Calciumhttps://www.cancer.gov/about-cancer/treatment/drugs/leucovorincalcium
- NCI Investigational Drugs
- NIPH Clinical Trials Search of Japan
- NLM RxNorm TerminologyLICENSEThe RxNorm Terminology is created by the National Library of Medicine (NLM) and is in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from NLM. Credit to the U.S. National Library of Medicine as the source is appreciated but not required. The full RxNorm dataset requires a free license.https://www.nlm.nih.gov/research/umls/rxnorm/docs/termsofservice.htmlleucovorin calciumhttps://rxnav.nlm.nih.gov/id/rxnorm/225852levoleucovorin calciumhttps://rxnav.nlm.nih.gov/id/rxnorm/979509
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/NORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- WHO Anatomical Therapeutic Chemical (ATC) ClassificationLICENSEUse of all or parts of the material requires reference to the WHO Collaborating Centre for Drug Statistics Methodology. Copying and distribution for commercial purposes is not allowed. Changing or manipulating the material is not allowed.https://www.whocc.no/copyright_disclaimer/Calcium folinatehttps://www.whocc.no/atc_ddd_index/?code=V03AF03
- Wikidataleucovorin calciumhttps://www.wikidata.org/wiki/Q27077063
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlVitamin B Complexhttps://www.ncbi.nlm.nih.gov/mesh/68014803
- PubChem
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html

 CID 5460341 (Calcium)
CID 5460341 (Calcium)