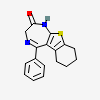
Bentazepam
- Bentazepam
- 29462-18-8
- Thiadipone
- Tiadipone
- CI-718
- Create:2019-01-15
- Modify:2025-01-04
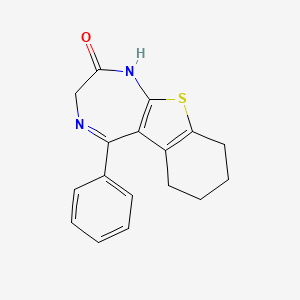
- 6,7-tetramethylene-5-phenyl-1,2-dihydro-3H-thieno(2,3-e)(1,4)diazepin-2-one
- bentazepam
- bentazepam sulfate, (1:1) salt
- CI 718
- CI-718
- QM 6008
- QM-6008
- Thiadipone
- Tiadipona
- Tiadipone
- Bentazepam
- 29462-18-8
- Thiadipone
- Tiadipone
- CI-718
- QM-6008
- 1,3,6,7,8,9-Hexahydro-5-phenyl-2H-(1)benzothieno(2,3-e)-1,4-diazepin-2-one
- MLS001240209
- 66JKK43S1Z
- SMR000674576
- 2H-(1)BENZOTHIENO(2,3-e)-1,4-DIAZEPIN-2-ONE, 1,3,6,7,8,9-HEXAHYDRO-5-PHENYL-
- Bentazepamum
- Bentazepam (1mg/mL in acetonitrile)
- Bentazepam [USAN:INN]
- 5-Phenyl-1,2,3,4,7,9-hexahydro-10-thia-6,9-diaza-benzo[a]azulen-8-one
- 5-phenyl-1,3,6,7,8,9-hexahydro-[1]benzothiolo[2,3-e][1,4]diazepin-2-one
- Bentazepamum [INN-Latin]
- CI 718
- BRN 1083088
- UNII-66JKK43S1Z
- Tiadipona
- 1,3,6,7,8,9-Hexahydro-5-phenyl-2H-[1]benzothieno[2,3-E]-1,4-diazepin-2-one
- BENTAZEPAM [INN]
- Bentazepam (USAN/INN)
- BENTAZEPAM [USAN]
- 6,7-Tetramethylene-5-phenyl-1,2-dihydro-3H-thieno(2,3-e)(1,4)diazepin-2-one
- BENTAZEPAM [MART.]
- BENTAZEPAM [WHO-DD]
- Oprea1_231952
- SCHEMBL44343
- cid_34592
- SCHEMBL1648551
- CHEMBL1521495
- BDBM70137
- DTXSID40897431
- CHEBI:135252
- HMS2233H05
- HMS3370L02
- MFCD00868003
- 5-phenyl-3,4,6,7,8,9-hexahydro-[1]benzothiolo[2,3-e][1,4]diazepin-2-one
- 5-phenyl-3,4,6,7,8,9-hexahydrobenzothiopheno[2,3-e][1,4]diazepin-2-one
- AKOS000707556
- DB14719
- 5-Phenyl-6,7,8,9-tetrahydro-1H-benzo[4,5]thieno[2,3-e][1,4]diazepin-2(3H)-one
- NCGC00244971-01
- DA-71375
- SY270603
- NS00114140
- D03083
- AB00876242-04
- Q1421936
- BRD-K89490285-001-08-1


H317 (100%): May cause an allergic skin reaction [Warning Sensitization, Skin]
H413 (100%): May cause long lasting harmful effects to aquatic life [Hazardous to the aquatic environment, long-term hazard]
P261, P272, P273, P280, P302+P352, P321, P333+P317, P362+P364, and P501
(The corresponding statement to each P-code can be found at the GHS Classification page.)
Skin Sens. 1 (100%)
Aquatic Chronic 4 (100%)
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=AIZFEOPQVZBNGH-UHFFFAOYSA-N
- Burnham Center for Chemical Genomics
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/
- ChemIDplusBentazepam [USAN:INN]https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0029462188ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- DrugBankLICENSECreative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode)https://www.drugbank.ca/legal/terms_of_useBentazepamhttps://www.drugbank.ca/drugs/DB14719
- EPA DSSToxCompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-notice5-phenyl-3,4,6,7,8,9-hexahydro-[1]benzothiolo[2,3-e][1,4]diazepin-2-onehttps://echa.europa.eu/substance-information/-/substanceinfo/100.123.6595-phenyl-3,4,6,7,8,9-hexahydro-[1]benzothiolo[2,3-e][1,4]diazepin-2-one (EC: 608-362-7)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/86609
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- ChEBI
- Open TargetsLICENSEDatasets generated by the Open Targets Platform are freely available for download.https://platform-docs.opentargets.org/licence
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.htmlChEMBL Protein Target Treehttps://www.ebi.ac.uk/chembl/g/#browse/targets
- Drug Gene Interaction database (DGIdb)LICENSEThe data used in DGIdb is all open access and where possible made available as raw data dumps in the downloads section.http://www.dgidb.org/downloadsBENTAZEPAMhttps://www.dgidb.org/drugs/ncit:C74189
- Therapeutic Target Database (TTD)
- Human Metabolome Database (HMDB)LICENSEHMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications.http://www.hmdb.ca/citing
- Japan Chemical Substance Dictionary (Nikkaji)
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.htmlAnatomical Therapeutic Chemical (ATC) classificationhttp://www.genome.jp/kegg-bin/get_htext?br08303.keg
- Metabolomics Workbench
- NCI Thesaurus (NCIt)LICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuseNCI Thesaurushttps://ncit.nci.nih.gov
- SpectraBase
- Springer Nature
- WHO Anatomical Therapeutic Chemical (ATC) ClassificationLICENSEUse of all or parts of the material requires reference to the WHO Collaborating Centre for Drug Statistics Methodology. Copying and distribution for commercial purposes is not allowed. Changing or manipulating the material is not allowed.https://www.whocc.no/copyright_disclaimer/
- WikipediaBentazepamhttps://en.wikipedia.org/wiki/Bentazepam
- PubChem
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlHypnotics and Sedativeshttps://www.ncbi.nlm.nih.gov/mesh/68006993
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/NORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 394398279https://pubchem.ncbi.nlm.nih.gov/substance/394398279
- NCBI