Aluminium selenide
- Aluminum selenide
- 1302-82-5
- Aluminum selenide (Al2Se3)
- Aluminium selenide
- dialuminum;selenium(2-)
- Create:2005-08-08
- Modify:2025-01-11
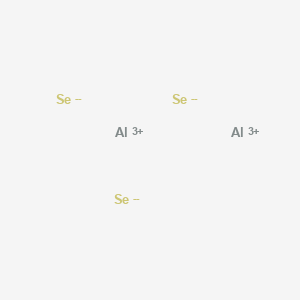
- Aluminum selenide
- 1302-82-5
- Aluminum selenide (Al2Se3)
- Aluminium selenide
- dialuminum;selenium(2-)
- 4R7PMP996Q
- DIALUMINUM TRISELENIDE
- UNII-4R7PMP996Q
- ALUMINUM SESQUISELENIDE
- ALUMINUM SELENIDE [MI]
- DTXSID80894848
- EINECS 215-110-6
- ALUMINUMSELENIDE
- CYRGZAAAWQRSMF-UHFFFAOYSA-N
- dialuminium(3+) ion triselandiide
- DTXCID201324411
- Q2634282

H301 (100%): Toxic if swallowed [Danger Acute toxicity, oral]
H331 (100%): Toxic if inhaled [Danger Acute toxicity, inhalation]
H373 (100%): May causes damage to organs through prolonged or repeated exposure [Warning Specific target organ toxicity, repeated exposure]
H400 (100%): Very toxic to aquatic life [Warning Hazardous to the aquatic environment, acute hazard]
H410 (100%): Very toxic to aquatic life with long lasting effects [Warning Hazardous to the aquatic environment, long-term hazard]
P260, P261, P264, P270, P271, P273, P301+P316, P304+P340, P316, P319, P321, P330, P391, P403+P233, P405, and P501
(The corresponding statement to each P-code can be found at the GHS Classification page.)
Aggregated GHS information provided per 4 reports by companies from 1 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies.
Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown.
- ChemIDplusChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- EPA Chemicals under the TSCAAluminum selenide (Al2Se3)https://www.epa.gov/chemicals-under-tscaEPA TSCA Classificationhttps://www.epa.gov/tsca-inventory
- EPA DSSToxAluminum selenidehttps://comptox.epa.gov/dashboard/DTXSID80894848CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-noticeAluminium selenidehttps://echa.europa.eu/substance-information/-/substanceinfo/100.013.737Aluminium selenide (EC: 215-110-6)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/106383
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingALUMINUM SELENIDEhttps://gsrs.ncats.nih.gov/ginas/app/beta/substances/4R7PMP996Q
- Toxin and Toxin Target Database (T3DB)LICENSET3DB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (T3DB) and the original publication.http://www.t3db.ca/downloadsAluminium selenidehttp://www.t3db.ca/toxins/T3D1828
- Wikidataaluminum selenidehttps://www.wikidata.org/wiki/Q2634282
- WikipediaSebacoyl chloridehttps://en.wikipedia.org/wiki/Sebacoyl_chlorideAluminium selenidehttps://en.wikipedia.org/wiki/Aluminium_selenide
- PubChem
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- EPA Substance Registry ServicesEPA SRS List Classificationhttps://sor.epa.gov/sor_internet/registry/substreg/LandingPage.do
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/

 CID 107674 (Selenide)
CID 107674 (Selenide) CID 5359268 (Aluminum)
CID 5359268 (Aluminum)