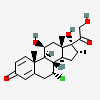
Alclometasone
- alclometasone
- 67452-97-5
- Aclometasone
- Alclometasone [INN:BAN]
- 7alpha-Chloro-16alpha-methylprednisolone
- Create:2005-12-16
- Modify:2025-01-04
 Alclometasone Dipropionate (active moiety of).
Alclometasone Dipropionate (active moiety of).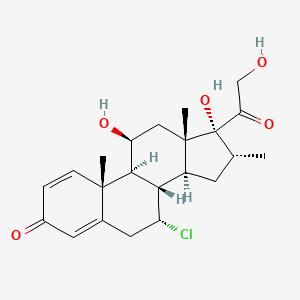
- alclometasone
- 67452-97-5
- Aclometasone
- Alclometasone [INN:BAN]
- 7alpha-Chloro-16alpha-methylprednisolone
- UNII-136H45TB7B
- 136H45TB7B
- Alclometasone (INN)
- CHEBI:53776
- (7R,8S,9S,10R,11S,13S,14S,16R,17R)-7-chloro-11,17-dihydroxy-17-(2-hydroxyacetyl)-10,13,16-trimethyl-7,8,9,11,12,14,15,16-octahydro-6H-cyclopenta[a]phenanthren-3-one
- 7alpha-Chloro-16alpha-methyl Prednisolone
- (7alpha,11beta,16alpha)-7-chloro-11,17,21-trihydroxy-16-methylpregna-1,4-diene-3,20-dione
- ALCLOMETASONE [INN]
- alclometasona
- alclometasonum
- ALCLOMETASONE [MI]
- SCHEMBL4127
- ALCLOMETASONE [VANDF]
- ALCLOMETASONE [WHO-DD]
- CHEMBL1201361
- 7a-hloro-16a-ethyl prednisolone
- D07AB10
- DTXSID60867277
- S01BA10
- HY-A0150
- AKOS040741062
- DB00240
- DA-60881
- CS-0017475
- NS00071906
- D07116
- EN300-25042268
- Q4713192
- (1R,2R,3aS,3bS,4R,9aR,9bS,10S,11aS)-4-chloro-1,10-dihydroxy-1-(2-hydroxyacetyl)-2,9a,11a-trimethyl-1H,2H,3H,3aH,3bH,4H,5H,7H,9aH,9bH,10H,11H,11aH-cyclopenta[a]phenanthren-7-one
- (1S,2R,9R,10S,11S,13R,14R,15S,17S)-9-chloro-14,17-dihydroxy-14-(2-hydroxyacetyl)-2,13,15-trimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadeca-3,6-dien-5-one
- (7.ALPHA.,11.BETA.,16.ALPHA.)-7-CHLORO-11,17,21-TRIHYDROXY-16-METHYLPREGNA-1,4-DIENE-3,20-DIONE
- PREGNA-1,4-DIENE-3,20-DIONE, 7-CHLORO-11,17,21-TRIHYDROXY-16-METHYL-, (7.ALPHA.,11.BETA.,16.ALPHA.)-
- Pregna-1,4-diene-3,20-dione, 7-chloro-11,17,21-trihydroxy-16-methyl-, (7a,11ss,16a)-; (7a,11ss,16a)-7-Chloro-11,17,21-trihydroxy-16-methylpregna-1,4-diene-3,20-dione; 7a-Chloro-16a-methylprednisolone; Alclometasone; Alclomethasone
- PREGNA-1,4-DIENE-3,20-DIONE, 7-CHLORO-11,17,21-TRIHYDROXY-16-METHYL-, (7alpha,11beta,16alpha)-
 Alclometasone Dipropionate (active moiety of)
Alclometasone Dipropionate (active moiety of)- Cytoplasm
- Extracellular
- Membrane
◉ Summary of Use during Lactation
Alclometasone has not been studied during breastfeeding. Since only extensive application of the most potent of these drugs cause systemic effects in the mother, it is unlikely that short-term application of topical corticosteroids would pose a risk to the breastfed infant by passage into breastmilk. However, it would be prudent to use the least potent drug on the smallest area of skin possible. It is particularly important to ensure that the infant's skin does not come into direct contact with the areas of skin that have been treated. Only the lower potency corticosteroids (e.g., hydrocortisone, triamcinolone) should be used on the nipple or areola where the infant could directly ingest the drugs from the skin. Only water-miscible cream or gel products should be applied to the breast because ointments may expose the infant to high levels of mineral paraffins via licking. Any topical corticosteroid should be wiped off thoroughly prior to nursing if it is being applied to the breast or nipple area.
◉ Effects in Breastfed Infants
Topical application of a corticosteroid with relatively high mineralocorticoid activity (isofluprednone acetate) to the mother's nipples resulted in prolonged QT interval, cushingoid appearance, severe hypertension, decreased growth and electrolyte abnormalities in her 2-month-old breastfed infant. The mother had used the cream since birth for painful nipples.
◉ Effects on Lactation and Breastmilk
Relevant published information was not found as of the revision date.
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=FJXOGVLKCZQRDN-PHCHRAKRSA-N
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/
- ChemIDplusAlclometasone [INN:BAN]https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0067452975ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- DrugBankLICENSECreative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode)https://www.drugbank.ca/legal/terms_of_useAlclometasonehttps://www.drugbank.ca/drugs/DB00240
- EPA DSSToxAlclometasonehttps://comptox.epa.gov/dashboard/DTXSID60867277CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- Human Metabolome Database (HMDB)LICENSEHMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications.http://www.hmdb.ca/citingAlclometasonehttp://www.hmdb.ca/metabolites/HMDB0014385
- ChEBI
- FDA Pharm ClassesLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingFDA Pharmacological Classificationhttps://www.fda.gov/ForIndustry/DataStandards/StructuredProductLabeling/ucm162549.htm
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.html
- ClinicalTrials.govLICENSEThe ClinicalTrials.gov data carry an international copyright outside the United States and its Territories or Possessions. Some ClinicalTrials.gov data may be subject to the copyright of third parties; you should consult these entities for any additional terms of use.https://clinicaltrials.gov/ct2/about-site/terms-conditions#Use
- Drug Gene Interaction database (DGIdb)LICENSEThe data used in DGIdb is all open access and where possible made available as raw data dumps in the downloads section.http://www.dgidb.org/downloadsALCLOMETASONEhttps://www.dgidb.org/drugs/rxcui:108088
- Therapeutic Target Database (TTD)Alclometasonehttps://idrblab.net/ttd/data/drug/details/D0F1EX
- Drugs and Lactation Database (LactMed)Alclometasonehttps://www.ncbi.nlm.nih.gov/books/n/lactmed/LM413/
- Japan Chemical Substance Dictionary (Nikkaji)
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.htmlAnatomical Therapeutic Chemical (ATC) classificationhttp://www.genome.jp/kegg-bin/get_htext?br08303.kegTarget-based classification of drugshttp://www.genome.jp/kegg-bin/get_htext?br08310.keg
- Metabolomics Workbench
- NCI Thesaurus (NCIt)LICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuseNCI Thesaurushttps://ncit.nci.nih.gov
- NLM RxNorm TerminologyLICENSEThe RxNorm Terminology is created by the National Library of Medicine (NLM) and is in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from NLM. Credit to the U.S. National Library of Medicine as the source is appreciated but not required. The full RxNorm dataset requires a free license.https://www.nlm.nih.gov/research/umls/rxnorm/docs/termsofservice.htmlalclometasonehttps://rxnav.nlm.nih.gov/id/rxnorm/108088
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/AclovateNORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- Springer Nature
- WHO Anatomical Therapeutic Chemical (ATC) ClassificationLICENSEUse of all or parts of the material requires reference to the WHO Collaborating Centre for Drug Statistics Methodology. Copying and distribution for commercial purposes is not allowed. Changing or manipulating the material is not allowed.https://www.whocc.no/copyright_disclaimer/Alclometasonehttps://www.whocc.no/atc_ddd_index/?code=S01BA10Alclometasonehttps://www.whocc.no/atc_ddd_index/?code=D07AB10
- Wikidataalclometasonehttps://www.wikidata.org/wiki/Q4713192
- WikipediaRivaroxabanhttps://en.wikipedia.org/wiki/RivaroxabanAlclometasonehttps://en.wikipedia.org/wiki/Alclometasone
- PubChem
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 388576778https://pubchem.ncbi.nlm.nih.gov/substance/388576778
- NCBI