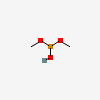Dimethyl ester phosphorous acid
PubChem CID
94853
Molecular Formula
Synonyms
- Phosphorous acid, dimethyl ester
- 96-36-6
- Methyl phosphite, (MeO)2(HO)P
- 237JNK8KUO
- Dimethyl ester phosphorous acid
Molecular Weight
110.05 g/mol
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Dates
- Create:2005-03-26
- Modify:2025-01-18
Description
Dimethyl Hydrogen Phosphite can cause cancer according to California Labor Code and the World Health Organization's International Agency for Research on Cancer (IARC).
Chemical Structure Depiction
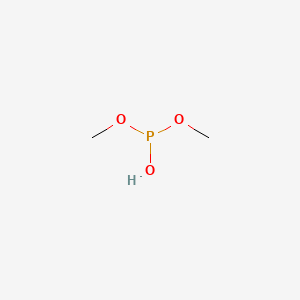
dimethyl hydrogen phosphite
Computed by Lexichem TK 2.7.0 (PubChem release 2021.10.14)
InChI=1S/C2H7O3P/c1-4-6(3)5-2/h3H,1-2H3
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
DLQDGVZAEYZNTG-UHFFFAOYSA-N
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
COP(O)OC
Computed by OEChem 2.3.0 (PubChem release 2024.12.12)
C2H7O3P
Computed by PubChem 2.2 (PubChem release 2021.10.14)
96-36-6
Property Name
Property Value
Reference
Property Name
Molecular Weight
Property Value
110.05 g/mol
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
XLogP3-AA
Property Value
-0.5
Reference
Computed by XLogP3 3.0 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Donor Count
Property Value
1
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Acceptor Count
Property Value
3
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Rotatable Bond Count
Property Value
2
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Exact Mass
Property Value
110.01328108 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Monoisotopic Mass
Property Value
110.01328108 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Topological Polar Surface Area
Property Value
38.7 Ų
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Heavy Atom Count
Property Value
6
Reference
Computed by PubChem
Property Name
Formal Charge
Property Value
0
Reference
Computed by PubChem
Property Name
Complexity
Property Value
28
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Isotope Atom Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Covalently-Bonded Unit Count
Property Value
1
Reference
Computed by PubChem
Property Name
Compound Is Canonicalized
Property Value
Yes
Reference
Computed by PubChem (release 2021.10.14)
Environmental transformation -> Pesticide transformation products (metabolite, successor)
S60 | SWISSPEST19 | Swiss Pesticides and Metabolites from Kiefer et al 2019 | DOI:10.5281/zenodo.3544759
Follow these links to do a live 2D search or do a live 3D search for this compound, sorted by annotation score. This section is deprecated (see here for details), but these live search links provide equivalent functionality to the table that was previously shown here.
Same Parent, Exact Count
Mixtures, Components, and Neutralized Forms Count
Similar Compounds (2D)
Similar Conformers (3D)
Dimethyl phosphonate (annotation moved to)
Dimethylphosphite (annotation moved to)
PubMed Count
Dimethyl phosphite is a known environmental transformation product of Phosmet.
S60 | SWISSPEST19 | Swiss Pesticides and Metabolites from Kiefer et al 2019 | DOI:10.5281/zenodo.3544759
Environmental transformation -> Pesticide transformation products (metabolite, successor)
S60 | SWISSPEST19 | Swiss Pesticides and Metabolites from Kiefer et al 2019 | DOI:10.5281/zenodo.3544759
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=DLQDGVZAEYZNTG-UHFFFAOYSA-N
- California Office of Environmental Health Hazard Assessment (OEHHA)Dimethyl Hydrogen Phosphitehttps://oehha.ca.gov/proposition-65/chemicals/dimethyl-hydrogen-phosphite
- ChemIDplusDimethyl ester phosphorous acidhttps://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000096366ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- DTP/NCILICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuseMethyl phosphite, (MeO)2(HO)Phttps://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=5271
- EPA DSSToxDimethyl hydrogen phosphitehttps://comptox.epa.gov/dashboard/DTXSID20242066CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingDIMETHYL ESTER PHOSPHOROUS ACIDhttps://gsrs.ncats.nih.gov/ginas/app/beta/substances/237JNK8KUO
- Japan Chemical Substance Dictionary (Nikkaji)
- NIST Mass Spectrometry Data CenterLICENSEData covered by the Standard Reference Data Act of 1968 as amended.https://www.nist.gov/srd/public-lawPhosporous acid, dimethyl esterhttp://www.nist.gov/srd/nist1a.cfm
- SpectraBaseDimethyl hydrogen phosphitehttps://spectrabase.com/spectrum/FDMocQlX5WeDimethyl hydrogen phosphitehttps://spectrabase.com/spectrum/CJB2llCHO27PHOSPHOROUS ACID, DIMETHYL ESTERhttps://spectrabase.com/spectrum/JCqVsYE9ovgPhosphorous acid, dimethyl esterhttps://spectrabase.com/spectrum/Cx6HH9wPLQ0Phosphonic acid, dimethyl esterhttps://spectrabase.com/spectrum/8vsuyay8bCXPhosphonic acid, dimethyl esterhttps://spectrabase.com/spectrum/AwJO5EOJH7l
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/NORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- Springer Nature
- Thieme ChemistryLICENSEThe Thieme Chemistry contribution within PubChem is provided under a CC-BY-NC-ND 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc-nd/4.0/
- WikidataPhosphorous acid, dimethyl esterhttps://www.wikidata.org/wiki/Q83125653
- PubChem
- CAMEO ChemicalsLICENSECAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data.https://cameochemicals.noaa.gov/help/reference/terms_and_conditions.htm?d_f=falseCAMEO Chemical Reactivity Classificationhttps://cameochemicals.noaa.gov/browse/react
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 403419248https://pubchem.ncbi.nlm.nih.gov/substance/403419248
- NCBI
CONTENTS