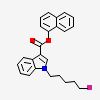
Naphthalen-1-yl 1-(5-fluoropentyl)-1h-indole-3-carboxylate
PubChem CID
91864534
Chemical Safety
Molecular Formula
Synonyms
- NM-2201
- 2042201-16-9
- NM2201
- CBL-2201
- naphthalen-1-yl 1-(5-fluoropentyl)-1h-indole-3-carboxylate
Molecular Weight
375.4 g/mol
Computed by PubChem 2.1 (PubChem release 2021.05.07)
Dates
- Create:2015-09-11
- Modify:2024-12-28
Description
Naphthalen-1-yl 1-(5-fluoropentyl)-1H-indole-3-carboxylate is a DEA Schedule I controlled substance. Substances in the DEA Schedule I have no currently accepted medical use in the United States, a lack of accepted safety for use under medical supervision, and a high potential for abuse. It is a Hallucinogenic substances substance.
Chemical Structure Depiction
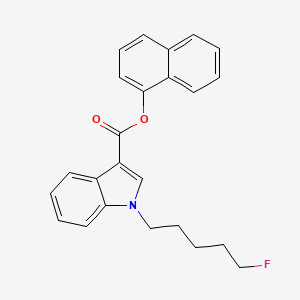
naphthalen-1-yl 1-(5-fluoropentyl)indole-3-carboxylate
Computed by Lexichem TK 2.7.0 (PubChem release 2021.05.07)
InChI=1S/C24H22FNO2/c25-15-6-1-7-16-26-17-21(20-12-4-5-13-22(20)26)24(27)28-23-14-8-10-18-9-2-3-11-19(18)23/h2-5,8-14,17H,1,6-7,15-16H2
Computed by InChI 1.0.6 (PubChem release 2021.05.07)
PRGFSQYZCKCBQO-UHFFFAOYSA-N
Computed by InChI 1.0.6 (PubChem release 2021.05.07)
C1=CC=C2C(=C1)C=CC=C2OC(=O)C3=CN(C4=CC=CC=C43)CCCCCF
Computed by OEChem 2.3.0 (PubChem release 2021.05.07)
C24H22FNO2
Computed by PubChem 2.1 (PubChem release 2021.05.07)
112-236-2
7221 (DEA schedule I controlled substance)
- CBL-2201
- napht-1-yl 1-(5-fluoropentyl)-1H-indole-3-carboxylate
- NM-2201
- 2042201-16-9
- NM2201
- CBL-2201
- naphthalen-1-yl 1-(5-fluoropentyl)-1h-indole-3-carboxylate
- CCQ6IR3CU2
- 5-Fluoro-sdb-005 indole
- CBL2201
- NAPHTHALEN-1-YL 1-(5-FLUOROPENTYL)INDOLE-3-CARBOXYLATE
- NM-2201 (1.0 mg/mL in Methanol)
- NM-2201; CBL-2201; Naphthalen-1-yl 1-(5-fluoropentyl)-1H-indol-3-carboxylate
- UNII-CCQ6IR3CU2
- DTXSID201009986
- NM 2201
- NS00017400
- Q20707163
- S900007150
- 1-Naphthalenyl 1-(5-fluoropentyl)-1H-indole-3-carboxylate
- naphthalen-1-yl1-(5-fluoropentyl)-1H-indole-3-carboxylate
- CBL-2201; Naphthalen-1-yl 1-(5-Fluoropentyl)-1H-indole-3-carboxylate
Property Name
Property Value
Reference
Property Name
Molecular Weight
Property Value
375.4 g/mol
Reference
Computed by PubChem 2.1 (PubChem release 2021.05.07)
Property Name
XLogP3-AA
Property Value
6
Reference
Computed by XLogP3 3.0 (PubChem release 2021.05.07)
Property Name
Hydrogen Bond Donor Count
Property Value
0
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.05.07)
Property Name
Hydrogen Bond Acceptor Count
Property Value
3
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.05.07)
Property Name
Rotatable Bond Count
Property Value
8
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.05.07)
Property Name
Exact Mass
Property Value
375.16345711 Da
Reference
Computed by PubChem 2.1 (PubChem release 2021.05.07)
Property Name
Monoisotopic Mass
Property Value
375.16345711 Da
Reference
Computed by PubChem 2.1 (PubChem release 2021.05.07)
Property Name
Topological Polar Surface Area
Property Value
31.2Ų
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.05.07)
Property Name
Heavy Atom Count
Property Value
28
Reference
Computed by PubChem
Property Name
Formal Charge
Property Value
0
Reference
Computed by PubChem
Property Name
Complexity
Property Value
513
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.05.07)
Property Name
Isotope Atom Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Covalently-Bonded Unit Count
Property Value
1
Reference
Computed by PubChem
Property Name
Compound Is Canonicalized
Property Value
Yes
Reference
Computed by PubChem (release 2012.11.26)
Pharmaceuticals -> Synthetic Cannabinoids or Psychoactive Compounds
S58 | PSYCHOCANNAB | Synthetic Cannabinoids and Psychoactive Compounds | DOI:10.5281/zenodo.3247723
Instrument Name
Bio-Rad FTS
Technique
ATR-Neat (DuraSamplIR II)
Source of Spectrum
Forensic Spectral Research
Source of Sample
Cayman Chemical Company
Catalog Number
<a href=https://www.caymanchem.com/product/15334>15334</a>
Lot Number
0459249-3
Copyright
Copyright © 2014-2024 John Wiley & Sons, Inc. All Rights Reserved.
Instrument Name
HP 5890 Series II / HP 5965B (GC/IRD)
Technique
Vapor Phase
Source of Spectrum
Forensic Spectral Research
Source of Sample
Cayman Chemical Company
Catalog Number
<a href=https://www.caymanchem.com/product/15334>15334</a>
Lot Number
0459249-3
Copyright
Copyright © 2022-2024 John Wiley & Sons, Inc. All Rights Reserved.
Follow these links to do a live 2D search or do a live 3D search for this compound, sorted by annotation score. This section is deprecated (see here for details), but these live search links provide equivalent functionality to the table that was previously shown here.
Similar Compounds (2D)
Similar Conformers (3D)
Same Count
Substance
Naphthalen-1-yl 1-(5-fluoropentyl)-1H-indole-3-carboxylate
Synonym(s)
NM2201|CBL2201
DEA Controlled Substances Code Number
7221
Controlled Substances Act Schedule
Schedule I - Substances in the DEA Schedule I have no currently accepted medical use in the United States, a lack of accepted safety for use under medical supervision, and a high potential for abuse.
Class
Hallucinogenic substances
EPA CPDat Chemical and Product Categories
The Chemical and Products Database, a resource for exposure-relevant data on chemicals in consumer products, Scientific Data, volume 5, Article number: 180125 (2018), DOI:10.1038/sdata.2018.125
Pictogram(s)

Signal
Warning
GHS Hazard Statements
H336 (100%): May cause drowsiness or dizziness [Warning Specific target organ toxicity, single exposure; Narcotic effects]
Precautionary Statement Codes
P261, P271, P304+P340, P319, P403+P233, P405, and P501
(The corresponding statement to each P-code can be found at the GHS Classification page.)
ECHA C&L Notifications Summary
The GHS information provided by 1 company from 1 notification to the ECHA C&L Inventory.
STOT SE 3 (100%)
DEA Controlled Substances
DEA schedule I controlled substance
21 CFR Sections 1308.11-1308.15 https://www.ecfr.gov/current/title-21/chapter-II/part-1308
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=PRGFSQYZCKCBQO-UHFFFAOYSA-N
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/1-Naphthalenyl 1-(5-fluoropentyl)-1H-indole-3-carboxylatehttps://commonchemistry.cas.org/detail?cas_rn=2042201-16-9
- ChemIDplusChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- EPA DSSTox1-Naphthalenyl 1-(5-fluoropentyl)-1H-indole-3-carboxylatehttps://comptox.epa.gov/dashboard/DTXSID201009986CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-notice1-(5-fluoropentyl)-1H-Indole-3-carboxylic acid-1-naphthalenyl esterhttps://echa.europa.eu1-(5-fluoropentyl)-1H-Indole-3-carboxylic acid-1-naphthalenyl ester (EC: 112-236-2)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/398213
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- Comparative Toxicogenomics Database (CTD)LICENSEIt is to be used only for research and educational purposes. Any reproduction or use for commercial purpose is prohibited without the prior express written permission of NC State University.http://ctdbase.org/about/legal.jspnapht-1-yl 1-(5-fluoropentyl)-1H-indole-3-carboxylatehttps://ctdbase.org/detail.go?type=chem&acc=C000611223
- Drug Enforcement Administration (DEA)LICENSEUnless otherwise indicated, information on Department of Justice websites is in the public domain and may be copied and distributed without permission. Citation of the Department of Justice as source of the information is appreciated, as appropriate.https://www.justice.gov/legalpoliciesNaphthalen-1-yl 1-(5-fluoropentyl)-1H-indole-3-carboxylatehttps://www.deadiversion.usdoj.gov/schedules/DEA drug and chemical classificationhttps://www.dea.gov/drug-information/drug-scheduling
- EPA Chemical and Products Database (CPDat)EPA CPDat Classificationhttps://www.epa.gov/chemical-research/chemical-and-products-database-cpdat
- Japan Chemical Substance Dictionary (Nikkaji)
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/NM-2201NORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- SpectraBaseNAPHTHALEN-1-YL-1-(5-FLUOROPENTYL)-1H-INDOLE-3-CARBOXYLATE;CBL-2202;NM-2201https://spectrabase.com/spectrum/8QpQ5XzvpFS
- Springer Nature
- Wikidata
- Wikipedia
- PubChemPFAS and Fluorinated Compounds in PubChemhttps://gitlab.com/uniluxembourg/lcsb/eci/pubchem-docs/-/raw/main/pfas-tree/PFAS_Tree.pdf?inline=false
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlnapht-1-yl 1-(5-fluoropentyl)-1H-indole-3-carboxylatehttps://www.ncbi.nlm.nih.gov/mesh/2015621
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 459391844https://pubchem.ncbi.nlm.nih.gov/substance/459391844
- NCBI
CONTENTS