1-(9-Fluorenyl)methyl chloroformate
- 28920-43-6
- 9-Fluorenylmethyl chloroformate
- Fmoc-Cl
- Fmoc chloride
- Fmoc-chloride
- Create:2005-03-27
- Modify:2025-01-18
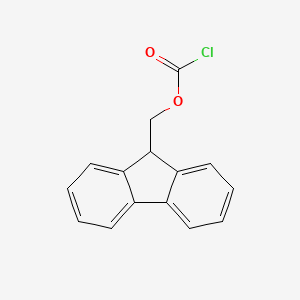
- 1-(9-fluorenyl)methyl chloroformate
- 9-FLMC
- 9-fluorenylmethoxycarbonyl chloride
- 9-fluorenylmethyl chloroformate
- 9-fluorenylmethylchloroformate
- FMOC-Cl
- 28920-43-6
- 9-Fluorenylmethyl chloroformate
- Fmoc-Cl
- Fmoc chloride
- Fmoc-chloride
- Carbonochloridic acid, 9H-fluoren-9-ylmethyl ester
- (9h-fluoren-9-yl)methyl carbonochloridate
- 9H-Fluoren-9-ylmethyl chloroformate
- 9-Fluorenylmethoxycarbonyl chloride
- 1-(9-Fluorenyl)methyl chloroformate
- 9-fluorenylmethylchloroformate
- Fluoren-9-ylmethyl chloroformate
- 9H-fluoren-9-ylmethyl carbonochloridate
- (9H-Fluoren-9-ylmethoxy)carbonyl Chloride
- (9H-fluoren-9-yl)methyl chloroformate
- CCRIS 2608
- Chloroformic Acid 9-Fluorenylmethyl Ester
- UNII-9PLB0BTT90
- EINECS 249-313-6
- 9PLB0BTT90
- MFCD00001138
- BRN 2279177
- 9-fluorenylmethyl chlorocarbonate
- FORMIC ACID, CHLORO-, FLUOREN-9-YLMETHYL ESTER
- (9H-fluoren-9-yl)methylcarbonochloridate
- DTXSID10183116
- Carbonochloridic acid 9H-fluoren-9-ylmethyl ester
- 9-FLUORENYLMETHYL CHLOROFORMATE [MI]
- 9-fluorenylmethyloxycarbonyl chloride
- FmocCl
- Fmoc chloride, 97%
- Fmoc-Cl (9-Fluorenylmethyl chloroformate)
- 9-FLMC
- SCHEMBL27547
- 9-fluorenemethyl chloroformate
- 9-fluorenylmethyl chlorformate
- 9-flourenylmethyl chloroformate
- 9-fluorenylmethyl-chloroformate
- 9-fluoreneylmethyl chloroformate
- (9-fluorenylmethyl) chloroformate
- DTXCID20105607
- 9-fluorenylmethoxycarbonyl-chloride
- 9-Fluorenylmethyoxycarbonyl chloride
- STR02408
- 9-Fluorenyl-methyloxycarbonyl chloride
- 9H-fluoren-9-ylmethylcarbonochloridate
- AKOS009157869
- AC-24425
- BP-21051
- 9H-Fluoren-9-ylmethyl chloridocarbonate #
- DB-004014
- Fmoc chloride, purum, >=98.0% (HPLC)
- F0197
- NS00028578
- EN300-37269
- C93459
- Fmoc chloride, BioReagent, >=99.0% (HPLC)
- FMOC-CL ChloroforMic acid 9-fluorenylMethyl ester
- Q827647
- Q-101295
- F0001-0905
- Fmoc chloride, for HPLC derivatization, >=99.0% (HPLC)
- Fmoc-Cl;9-Fluorenylmethoxycarbonyl-chloride; Fmoc-chloride
- 9-Fluorenylmethyl chloroformate; Chloroformic acid 9-fluorenylmethyl ester


H302 (17.5%): Harmful if swallowed [Warning Acute toxicity, oral]
H314 (100%): Causes severe skin burns and eye damage [Danger Skin corrosion/irritation]
H318 (11.2%): Causes serious eye damage [Danger Serious eye damage/eye irritation]
H332 (17.5%): Harmful if inhaled [Warning Acute toxicity, inhalation]
P260, P261, P264, P264+P265, P270, P271, P280, P301+P317, P301+P330+P331, P302+P361+P354, P304+P340, P305+P354+P338, P316, P317, P321, P330, P363, P405, and P501
(The corresponding statement to each P-code can be found at the GHS Classification page.)
Aggregated GHS information provided per 80 reports by companies from 13 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies.
Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown.
Acute Tox. 4 (17.5%)
Skin Corr. 1B (100%)
Eye Dam. 1 (11.2%)
Acute Tox. 4 (17.5%)
Status: Active Update: 28-05-2018 https://echa.europa.eu/registration-dossier/-/registered-dossier/25624
Status: Active Update: 29-03-2019 https://echa.europa.eu/registration-dossier/-/registered-dossier/28092
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=IRXSLJNXXZKURP-UHFFFAOYSA-N
- Australian Industrial Chemicals Introduction Scheme (AICIS)Carbonochloridic acid, 9H-fluoren-9-ylmethyl esterhttps://services.industrialchemicals.gov.au/search-assessments/Carbonochloridic acid, 9H-fluoren-9-ylmethyl esterhttps://services.industrialchemicals.gov.au/search-inventory/
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/9-Fluorenylmethyl chloroformatehttps://commonchemistry.cas.org/detail?cas_rn=28920-43-6
- ChemIDplus1-(9-Fluorenyl)methyl chloroformatehttps://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0028920436ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- EPA DSSTox1-(9-Fluorenyl)methyl chloroformatehttps://comptox.epa.gov/dashboard/DTXSID10183116CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-notice9H-fluoren-9-ylmethyl chloroformatehttps://chem.echa.europa.eu/100.044.8169H-fluoren-9-ylmethyl chloroformate (EC: 249-313-6)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/96607
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking1-(9-FLUORENYL)METHYL CHLOROFORMATEhttps://gsrs.ncats.nih.gov/ginas/app/beta/substances/9PLB0BTT90
- New Zealand Environmental Protection Authority (EPA)LICENSEThis work is licensed under the Creative Commons Attribution-ShareAlike 4.0 International licence.https://www.epa.govt.nz/about-this-site/general-copyright-statement/(9-Fluorenylmethyl) chloroformatehttps://www.epa.govt.nz/industry-areas/hazardous-substances/guidance-for-importers-and-manufacturers/hazardous-substances-databases/
- Human Metabolome Database (HMDB)LICENSEHMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications.http://www.hmdb.ca/citing9-Fluorenylmethyl chloroformatehttp://www.hmdb.ca/metabolites/HMDB0245743
- Japan Chemical Substance Dictionary (Nikkaji)
- NIST Mass Spectrometry Data CenterLICENSEData covered by the Standard Reference Data Act of 1968 as amended.https://www.nist.gov/srd/public-lawCarbonochloridic acid, 9H-fluoren-9-ylmethyl esterhttp://www.nist.gov/srd/nist1a.cfm
- SpectraBaseCarbonochloridic acid, 9H-fluoren-9-ylmethyl esterhttps://spectrabase.com/spectrum/5lxcvqcd4eGFMOC-Chloridehttps://spectrabase.com/spectrum/GCQWOJDwaSS9-Fluorenylmethylchloroformatehttps://spectrabase.com/spectrum/H9LOE4o3uooCHLOROFORMIC ACID, 9-FLUORENYLMETHYL ESTERhttps://spectrabase.com/spectrum/AAEsEcBbybgChloroformic acid, 9-fluorenylmethyl esterhttps://spectrabase.com/spectrum/IzwYQn207SVCHLOROFORMIC ACID, 9-FLUORENYLMETHYL ESTERhttps://spectrabase.com/spectrum/AFfTGojmYRB9-Fluorenylmethylchloroformatehttps://spectrabase.com/spectrum/6wp60wb9u9tCarbonochloridic acid, 9H-fluoren-9-ylmethyl esterhttps://spectrabase.com/spectrum/9Lj5hdxQ3NLCarbonochloridic acid, 9H-fluoren-9-ylmethyl esterhttps://spectrabase.com/spectrum/Nvk0LnaSu99-Fluorenylmethylchloroformatehttps://spectrabase.com/spectrum/1zctzawfZbrFMOC-Chloridehttps://spectrabase.com/spectrum/LnHWUy0yF0EFmoc chloridehttps://spectrabase.com/spectrum/H5LP1cwq9yc
- RCSB Protein Data Bank (RCSB PDB)LICENSEData files contained in the PDB archive (ftp://ftp.wwpdb.org) are free of all copyright restrictions and made fully and freely available for both non-commercial and commercial use. Users of the data should attribute the original authors of that structural data.https://www.rcsb.org/pages/policies
- Springer Nature
- Thieme ChemistryLICENSEThe Thieme Chemistry contribution within PubChem is provided under a CC-BY-NC-ND 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc-nd/4.0/
- Wikidata1-(9-fluorenyl)methyl chloroformatehttps://www.wikidata.org/wiki/Q827647
- Wiley
- PubChem
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.html1-(9-fluorenyl)methyl chloroformatehttps://www.ncbi.nlm.nih.gov/mesh/67054007Indicators and Reagentshttps://www.ncbi.nlm.nih.gov/mesh/68007202
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/NORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 388516893https://pubchem.ncbi.nlm.nih.gov/substance/388516893

