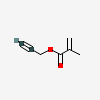
Propargyl methacrylate
- Propargyl methacrylate
- 13861-22-8
- 2-Propynyl methacrylate
- 2-Propenoic acid, 2-methyl-, 2-propyn-1-yl ester
- Prop-2-ynyl methacrylate
- Create:2005-08-08
- Modify:2025-01-11
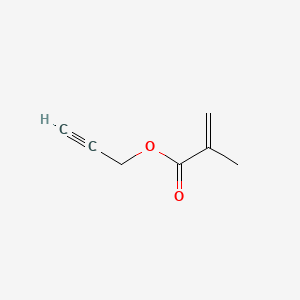
- Propargyl methacrylate
- 13861-22-8
- 2-Propynyl methacrylate
- 2-Propenoic acid, 2-methyl-, 2-propyn-1-yl ester
- Prop-2-ynyl methacrylate
- prop-2-ynyl 2-methylprop-2-enoate
- prop-2-yn-1-yl methacrylate
- U7MJ2445SD
- EINECS 237-599-5
- 2-Propenoic acid, 2-methyl-, 2-propynyl ester
- prop-2-yn-1-yl 2-methylprop-2-enoate
- AI3-37958
- DTXSID8065666
- MFCD00048123
- UNII-U7MJ2445SD
- SCHEMBL194435
- Methacrylic acid propargyl ester
- DTXCID9034494
- AKOS006229392
- AS-67944
- DB-042434
- NS00024487
- G77644
- Propargyl methacrylate, 98%, stab. with 250ppm 4-methoxyphenol


H226 (100%): Flammable liquid and vapor [Warning Flammable liquids]
H315 (100%): Causes skin irritation [Warning Skin corrosion/irritation]
H319 (100%): Causes serious eye irritation [Warning Serious eye damage/eye irritation]
H335 (100%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation]
P210, P233, P240, P241, P242, P243, P261, P264, P264+P265, P271, P280, P302+P352, P303+P361+P353, P304+P340, P305+P351+P338, P319, P321, P332+P317, P337+P317, P362+P364, P370+P378, P403+P233, P403+P235, P405, and P501
(The corresponding statement to each P-code can be found at the GHS Classification page.)
Aggregated GHS information provided per 6 reports by companies from 2 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies.
Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown.
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=PZAWASVJOPLHCJ-UHFFFAOYSA-N
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/Propargyl methacrylatehttps://commonchemistry.cas.org/detail?cas_rn=13861-22-82-Propenoic acid, 2-methyl-, 2-propyn-1-yl ester, homopolymerhttps://commonchemistry.cas.org/detail?cas_rn=30756-20-8
- ChemIDplusPropargyl methacrylatehttps://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0013861228ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- EPA Chemicals under the TSCA2-Propenoic acid, 2-methyl-, 2-propyn-1-yl esterhttps://www.epa.gov/chemicals-under-tscaEPA TSCA Classificationhttps://www.epa.gov/tsca-inventory
- EPA DSSTox2-Propynyl methacrylatehttps://comptox.epa.gov/dashboard/DTXSID8065666CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-noticeProp-2-ynyl methacrylatehttps://echa.europa.eu/substance-information/-/substanceinfo/100.034.167Prop-2-ynyl methacrylate (EC: 237-599-5)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/72610
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingPROPARGYL METHACRYLATEhttps://gsrs.ncats.nih.gov/ginas/app/beta/substances/U7MJ2445SD
- Japan Chemical Substance Dictionary (Nikkaji)
- NIST Mass Spectrometry Data CenterLICENSEData covered by the Standard Reference Data Act of 1968 as amended.https://www.nist.gov/srd/public-law2-Propenoic acid, 2-methyl-, 2-propynyl esterhttp://www.nist.gov/srd/nist1a.cfm
- SpectraBasePropargyl methacrylatehttps://spectrabase.com/spectrum/Jx7iyI0J9mpPROPARGYL METHACRYLATEhttps://spectrabase.com/spectrum/BGhjhI6r1u8Propargyl methacrylatehttps://spectrabase.com/spectrum/15Vt0oU20GlPROPARGYL METHACRYLATEhttps://spectrabase.com/spectrum/8b6GdNvInER
- Springer Nature
- Wikidata2-Propynyl methacrylatehttps://www.wikidata.org/wiki/Q81992311
- Wiley
- PubChem
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/NORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- EPA Substance Registry ServicesEPA SRS List Classificationhttps://sor.epa.gov/sor_internet/registry/substreg/LandingPage.do
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 403382330https://pubchem.ncbi.nlm.nih.gov/substance/403382330