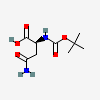
tert-Butoxycarbonylasparagine
- Boc-Asn-OH
- 7536-55-2
- Boc-L-asparagine
- Boc-Asn
- tert-Butoxycarbonylasparagine
- Create:2005-06-24
- Modify:2025-01-18
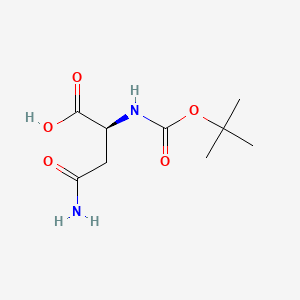

- Boc-Asn-OH
- 7536-55-2
- Boc-L-asparagine
- Boc-Asn
- tert-Butoxycarbonylasparagine
- N-(tert-Butoxycarbonyl)-L-asparagine
- Boc-L-Asn-OH
- Nalpha-Boc-L-asparagine
- tert-Butoxycarbonyl-L-asparagine
- N-(tert-Butoxycarbonyl)asparagine
- tert-Butyloxycarbonyl-L-asparagine
- N-alpha-Boc-L-asparagine
- MFCD00038152
- N(a)-tert-Butoxycarbonyl-L-asparagine
- N(2)-tert-Butoxycarbonyl-L-asparagine
- N2-((1,1-Dimethylethoxy)carbonyl)-L-asparagine
- L-Asparagine, N2-[(1,1-dimethylethoxy)carbonyl]-
- Asparagine, N(2)-carboxy-, N(2)-tert-butyl ester, L-
- CHEBI:3146
- (2S)-4-amino-2-[(2-methylpropan-2-yl)oxycarbonylamino]-4-oxobutanoic acid
- N(alpha)-t-butoxycarbonyl-L-asparagine
- N2-((tert-Butoxy)carbonyl)-L-asparagine
- N2-[(tert-butoxy)carbonyl]-L-asparagine
- 2-tert-Butoxycarbonylamino-succinamic acid
- L-Asparagine, N2-((1,1-dimethylethoxy)carbonyl)-
- C9H16N2O5
- BOC-(S)-2-AMINOSUCCINIC ACID 4-AMIDE MONOHYDRATE
- Nalpha-(tert-Butoxycarbonyl)-L-asparagine
- BOC-ASPARAGINE
- N-alpha-t-BOC-L-ASPARAGINE
- N-BOC-L-ASPARAGINE
- BocAsnOH
- EINECS 231-405-2
- Boc- Asn-OH
- NSC 154980
- N-a-t-Boc-asparagine
- SCHEMBL607848
- CHEMBL1222167
- DTXSID80884401
- N-tert-butoxycarbonyl-L-asparagine
- (tert-Butoxycarbonyl)-L-asparagine
- (2S)-2-{[(tert-butoxy)carbonyl]amino}-3-carbamoylpropanoic acid
- ALBB-028188
- N-tert-butyloxycarbonyl-L-asparagine
- STL466184
- AKOS012536057
- AKOS015922890
- Na-(tert-Butoxycarbonyl)-L-asparagine
- AC-8608
- Boc-Asn-OH, >=98.5% (T)
- CS-W019494
- DS-13747
- N(2)-(tert-butoxycarbonyl)-L-asparagine
- N~2~-(tert-butoxycarbonyl)-L-asparagine
- SY024209
- B1627
- NS00046354
- C01410
- EN300-186646
- M03264
- N-[(1,1-dimethylethoxy)carbonyl]-L-asparagine
- N-ALPHA-T-BUTYLOXYCARBONYL-L-ASPARAGINE
- J-300046
- Q-101639
- L-Asparagine, N(2)-[(1,1-dimethylethoxy)carbonyl]-
- L-asparagine, N~2~-[(1,1-dimethylethoxy)carbonyl]-
- Q27105952
- (S)-4-AMINO-2-(TERT-BUTOXYCARBONYLAMINO)-4-OXOBUTANOIC ACID
199.2 999
231 978
156.9 849
97 202
113 186
96.9 999
113.1 916
157 175
171.3 101
139.1 89

H315 (33.3%): Causes skin irritation [Warning Skin corrosion/irritation]
H319 (33.3%): Causes serious eye irritation [Warning Serious eye damage/eye irritation]
H335 (33.3%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation]
P261, P264, P264+P265, P271, P280, P302+P352, P304+P340, P305+P351+P338, P319, P321, P332+P317, P337+P317, P362+P364, P403+P233, P405, and P501
(The corresponding statement to each P-code can be found at the GHS Classification page.)
Aggregated GHS information provided per 3 reports by companies from 2 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies.
Reported as not meeting GHS hazard criteria per 2 of 3 reports by companies. For more detailed information, please visit ECHA C&L website.
There is 1 notification provided by 1 of 3 reports by companies with hazard statement code(s).
Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown.
Skin Irrit. 2 (33.3%)
Eye Irrit. 2A (33.3%)
STOT SE 3 (33.3%)
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=FYYSQDHBALBGHX-YFKPBYRVSA-N
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/tert-Butoxycarbonyl-L-asparaginehttps://commonchemistry.cas.org/detail?cas_rn=7536-55-2
- ChemIDplustert-Butoxycarbonylasparaginehttps://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0007536552ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- EPA Chemicals under the TSCAL-Asparagine, N2-[(1,1-dimethylethoxy)carbonyl]-https://www.epa.gov/chemicals-under-tscaEPA TSCA Classificationhttps://www.epa.gov/tsca-inventory
- EPA DSSToxL-Asparagine, N2-[(1,1-dimethylethoxy)carbonyl]-https://comptox.epa.gov/dashboard/DTXSID80884401CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-noticeN2-[(tert-butoxy)carbonyl]-L-asparaginehttps://echa.europa.eu/substance-information/-/substanceinfo/100.028.551N2-[(tert-butoxy)carbonyl]-L-asparagine (EC: 231-405-2)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/69529
- ChEBIN(alpha)-t-butoxycarbonyl-L-asparaginehttps://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:3146
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.htmlChEMBL Protein Target Treehttps://www.ebi.ac.uk/chembl/g/#browse/targets
- Japan Chemical Substance Dictionary (Nikkaji)
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.html
- MassBank EuropeN-a-t-Boc-asparaginehttps://massbank.eu/MassBank/Result.jsp?inchikey=FYYSQDHBALBGHX-YFKPBYRVSA-N
- MassBank of North America (MoNA)LICENSEThe content of the MoNA database is licensed under CC BY 4.0.https://mona.fiehnlab.ucdavis.edu/documentation/license
- NIST Mass Spectrometry Data CenterLICENSEData covered by the Standard Reference Data Act of 1968 as amended.https://www.nist.gov/srd/public-lawL-Asparagine, N(2)-[(1,1-dimethylethoxy)carbonyl]-http://www.nist.gov/srd/nist1a.cfm
- SpectraBase
- Springer Nature
- Thieme ChemistryLICENSEThe Thieme Chemistry contribution within PubChem is provided under a CC-BY-NC-ND 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc-nd/4.0/
- WikidataN(alpha)-t-butoxycarbonyl-L-asparaginehttps://www.wikidata.org/wiki/Q27105952
- PubChem
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/NORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- EPA Substance Registry ServicesEPA SRS List Classificationhttps://sor.epa.gov/sor_internet/registry/substreg/LandingPage.do
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 403033235https://pubchem.ncbi.nlm.nih.gov/substance/403033235