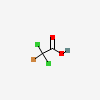
Bromodichloroacetic Acid
- Bromodichloroacetic acid
- 71133-14-7
- 2-bromo-2,2-dichloroacetic acid
- Dichlorobromoacetic acid
- Acetic acid,2-bromo-2,2-dichloro-
- Create:2005-08-08
- Modify:2025-01-18
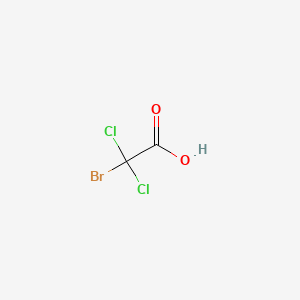
- bromodichloroacetate
- bromodichloroacetic acid
- Bromodichloroacetic acid
- 71133-14-7
- 2-bromo-2,2-dichloroacetic acid
- Dichlorobromoacetic acid
- Acetic acid,2-bromo-2,2-dichloro-
- Bromodichloroacetate
- Acetic acid, bromodichloro-
- UNII-SJ84PTP2HD
- SJ84PTP2HD
- DTXSID4024644
- HSDB 7620
- DTXCID404644
- BROMODICHLOROACETIC ACID [HSDB]
- ACETIC ACID, 2-BROMO-2,2-DICHLORO-
- BDCAA
- SCHEMBL752018
- CHEMBL453528
- XSWVFEQKZFUULO-UHFFFAOYSA-N
- Tox21_200923
- Acetic acid, 2-bromo-2,2-dichloro
- AT45180
- NCGC00091459-01
- NCGC00091459-02
- NCGC00258477-01
- CAS-71133-14-7
- DB-254107
- Bromodichloroacetic acid, analytical standard
- NS00001440
- Q27289240
- Bromdichloroacetic acid, vial of 1 g, analytical standard


H302 (100%): Harmful if swallowed [Warning Acute toxicity, oral]
H312 (100%): Harmful in contact with skin [Warning Acute toxicity, dermal]
H314 (100%): Causes severe skin burns and eye damage [Danger Skin corrosion/irritation]
H318 (92.7%): Causes serious eye damage [Danger Serious eye damage/eye irritation]
H332 (100%): Harmful if inhaled [Warning Acute toxicity, inhalation]
P260, P261, P264, P264+P265, P270, P271, P280, P301+P317, P301+P330+P331, P302+P352, P302+P361+P354, P304+P340, P305+P354+P338, P316, P317, P321, P330, P362+P364, P363, P405, and P501
(The corresponding statement to each P-code can be found at the GHS Classification page.)
Aggregated GHS information provided per 41 reports by companies from 2 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies.
Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown.
Acute Tox. 4 (100%)
Acute Tox. 4 (100%)
Skin Corr. 1B (100%)
Eye Dam. 1 (92.7%)
Acute Tox. 4 (100%)
Carcinogenicity - Category 1B
Specific target organ toxicity - Repeated exposure - Category 2 (liver, genetic organs (men), hematopoietic system)
Hazard Traits - Carcinogenicity
Authoritative List - Prop 65
Report - regardless of intended function of ingredient in the product
 Clear Evidence
Clear Evidence Clear Evidence
Clear Evidence Clear Evidence
Clear Evidence Clear Evidence
Clear EvidenceUnder the conditions of these 2-year studies, there was clear evidence of carcinogenic activity (see a summary of the Peer Review Panel comments and the public discussion on this Technical Report in Appendix P) of bromodichloroacetic acid in male F344/NTac rats based on increased incidences of malignant mesothelioma and the combined incidences of epithelial tumors of the skin. Occurrences of subcutaneous fibromas were also related to exposure to bromodichloroacetic acid. Occurrences of glioma or oligodendroglioma (combined) of the brain, squamous cell papilloma and squamous cell carcinoma of the oral cavity (oral mucosa or tongue), adenoma of the large intestine, and fibroadenoma of the mammary gland may have been related to exposure to bromodichloroacetic acid. There was clear evidence of carcinogenic activity of bromodichloroacetic acid in female F344/NTac rats based on increased incidences of fibroadenoma and carcinoma of the mammary gland. The occurrences of glioma or oligodendroglioma (combined) of the brain may have been related to bromodichloroacetic acid exposure. There was clear evidence of carcinogenic activity of bromodichloroacetic acid in male B6C3F1/N mice based on increased incidences of hepatocellular carcinoma and hepatoblastoma and increased incidences of adenoma or carcinoma (combined) of the Harderian gland. There was clear evidence of carcinogenic activity of bromodichloroacetic acid in female B6C3F1/N mice based on increased incidences of hepatocellular adenoma, hepatocellular carcinoma, and hepatoblastoma.
Exposure to bromodichloroacetic acid for 2 years resulted in increased incidences of nonneoplastic lesions in the bone marrow and liver of male and female rats, spleen of female rats, liver of male and female mice, and testis and epididymis of male mice.
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=XSWVFEQKZFUULO-UHFFFAOYSA-N
- California Office of Environmental Health Hazard Assessment (OEHHA)Bromodichloroacetic acidhttps://oehha.ca.gov/proposition-65/chemicals/bromodichloroacetic-acid
- California Safe Cosmetics Program (CSCP) Product DatabaseBromodichloroacetic acidhttps://www.cdph.ca.gov/Programs/CCDPHP/DEODC/OHB/CSCP/Pages/About-CSCP.aspx
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/Bromodichloroacetic acidhttps://commonchemistry.cas.org/detail?cas_rn=71133-14-7
- ChemIDplusBromodichloroacetic acidhttps://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0071133147ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- EPA DSSToxBromodichloroacetic acidhttps://comptox.epa.gov/dashboard/DTXSID4024644CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-noticeBromodichloroacetic acidhttps://echa.europa.eu/substance-information/-/substanceinfo/100.151.989Bromodichloroacetic acid (EC: 623-296-9)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/156588
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingBROMODICHLOROACETIC ACIDhttps://gsrs.ncats.nih.gov/ginas/app/beta/substances/SJ84PTP2HD
- Hazardous Substances Data Bank (HSDB)BROMODICHLOROACETIC ACIDhttps://pubchem.ncbi.nlm.nih.gov/source/hsdb/7620
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.html
- Drug Gene Interaction database (DGIdb)LICENSEThe data used in DGIdb is all open access and where possible made available as raw data dumps in the downloads section.http://www.dgidb.org/downloadsBROMODICHLOROACETIC ACIDhttps://www.dgidb.org/drugs/chembl:CHEMBL453528
- EPA Pesticide Ecotoxicity Database
- NITE-CMCBromodichloroacetic acid - FY2016 (New/original classication)https://www.chem-info.nite.go.jp/chem/english/ghs/16-mhlw-0015e.html
- Japan Chemical Substance Dictionary (Nikkaji)
- NTP Technical ReportsBromodichloroacetic Acidhttps://ntp.niehs.nih.gov/data/tr
- Springer Nature
- Wikidatabromodichloroacetic acidhttps://www.wikidata.org/wiki/Q27289240
- PubChem
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlbromodichloroacetic acidhttps://www.ncbi.nlm.nih.gov/mesh/67100580
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/NORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- EPA Substance Registry ServicesEPA SRS List Classificationhttps://sor.epa.gov/sor_internet/registry/substreg/LandingPage.do
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 389739725https://pubchem.ncbi.nlm.nih.gov/substance/389739725