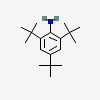
2,4,6-Tri-tert-butylaniline
- 2,4,6-Tri-tert-butylaniline
- 961-38-6
- 2,4,6-tritert-butylaniline
- 2,4,6-tri-t-Butylaniline
- 2,4,6-Tri(tert-butyl)aniline
- Create:2005-03-27
- Modify:2025-01-04
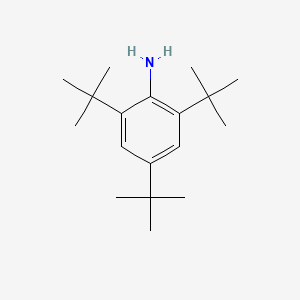
- 2,4,6-Tri-tert-butylaniline
- 961-38-6
- 2,4,6-tritert-butylaniline
- 2,4,6-tri-t-Butylaniline
- 2,4,6-Tri(tert-butyl)aniline
- Benzenamine, 2,4,6-tris(1,1-dimethylethyl)-
- MFCD00011645
- Aniline, tri-tert-butyl-
- 2,4,6-TRI-TERT-BUTYLPHENYLAMINE
- 2,4,6-tritert-butylphenylamine
- 2,4,6-Tri-tert-butyl-phenylamine
- Aniline, 2,4,6-tri-tert-butyl-
- EINECS 213-507-9
- (2,4,6-Tri-tert-butylphenyl)amine
- Oprea1_343921
- SCHEMBL139484
- 2,4,6-tris(tert.-butyl)anilin
- DTXSID30242118
- CHEBI:190998
- STK670835
- 2,4,6-Tri-tert-butylaniline, 99%
- AKOS002669419
- CS-W021499
- DS-0924
- AC-22746
- SY039915
- DB-103939
- 2 pound not4 pound not6-tri-t-Butylaniline
- EU-0017571
- NS00040474
- T1694
- AE-848/01459002
- SR-01000511057
- SR-01000511057-1
- F1945-0002
- 3-carboxy-4-[3-(diethylamino)-6-diethyliminio-xanthen-9-yl]benzoate
- InChI=1/C18H31N/c1-16(2,3)12-10-13(17(4,5)6)15(19)14(11-12)18(7,8)9/h10-11H,19H2,1-9H

H302 (11.4%): Harmful if swallowed [Warning Acute toxicity, oral]
H312 (11.4%): Harmful in contact with skin [Warning Acute toxicity, dermal]
H315 (100%): Causes skin irritation [Warning Skin corrosion/irritation]
H319 (100%): Causes serious eye irritation [Warning Serious eye damage/eye irritation]
H332 (11.4%): Harmful if inhaled [Warning Acute toxicity, inhalation]
H335 (97.7%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation]
P261, P264, P264+P265, P270, P271, P280, P301+P317, P302+P352, P304+P340, P305+P351+P338, P317, P319, P321, P330, P332+P317, P337+P317, P362+P364, P403+P233, P405, and P501
(The corresponding statement to each P-code can be found at the GHS Classification page.)
Aggregated GHS information provided per 44 reports by companies from 4 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies.
Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown.
Acute Tox. 4 (11.4%)
Acute Tox. 4 (11.4%)
Skin Irrit. 2 (100%)
Eye Irrit. 2 (100%)
Acute Tox. 4 (11.4%)
STOT SE 3 (97.7%)
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=REJGDSCBQPJPQT-UHFFFAOYSA-N
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/2,4,6-Tris(1,1-dimethylethyl)benzenaminehttps://commonchemistry.cas.org/detail?cas_rn=961-38-6
- ChemIDplus2,4,6-Tri-tert-butylanilinehttps://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000961386ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- EPA DSSTox2,4,6-Tri-tert-butylanilinehttps://comptox.epa.gov/dashboard/DTXSID30242118CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-notice2,4,6-tri-tert-butylanilinehttps://echa.europa.eu/substance-information/-/substanceinfo/100.012.2802,4,6-tri-tert-butylaniline (EC: 213-507-9)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/92662
- ChEBI2,4,6-tri-tert-butylanilinehttps://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:190998
- Crystallography Open Database (COD)LICENSEAll data in the COD and the database itself are dedicated to the public domain and licensed under the CC0 License. Users of the data should acknowledge the original authors of the structural data.https://creativecommons.org/publicdomain/zero/1.0/
- Japan Chemical Substance Dictionary (Nikkaji)
- NIST Mass Spectrometry Data CenterLICENSEData covered by the Standard Reference Data Act of 1968 as amended.https://www.nist.gov/srd/public-lawBenzenamine, 2,4,6-tris(1,1-dimethylethyl)-http://www.nist.gov/srd/nist1a.cfm
- NMRShiftDB
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/NORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- SpectraBase2,4,6-tri-tert-butylanilinehttps://spectrabase.com/spectrum/2msW5r36QVibenzenamine, 2,4,6-tris(1,1-dimethylethyl)-https://spectrabase.com/spectrum/LuKFuoGcbks2,4,6-TRI-tert-BUTYLANILINEhttps://spectrabase.com/spectrum/AL4CP7Z3DQ22,4,6-Tri-tert-butylanilinehttps://spectrabase.com/spectrum/BEvl1ODQgu9REJGDSCBQPJPQT-UHFFFAOYSA-Nhttps://spectrabase.com/spectrum/24fAOaJNZbu2,4,6-Tri-tert-butylanilinehttps://spectrabase.com/spectrum/E2p1L2fzEMi2,4,6-Tri-tert-butylanilinehttps://spectrabase.com/spectrum/KwjwDnlcdzb2,4,6-Tri-tert-butylanilinehttps://spectrabase.com/spectrum/3Me2jbJABPr2,4,6-Tri-tert-butylanilinehttps://spectrabase.com/spectrum/C7kVYyoO47H2,4,6-Tri-tert-butylanilinehttps://spectrabase.com/spectrum/93z3bRuGId02,4,6-Tri-tert-butylanilinehttps://spectrabase.com/spectrum/9NGDyoDUJFq
- Springer Nature
- SpringerMaterials2,4,6-Tri-tert-butyl-phenylaminehttps://materials.springer.com/substanceprofile/docs/smsid_vcolwrelzunsiiqj
- Thieme ChemistryLICENSEThe Thieme Chemistry contribution within PubChem is provided under a CC-BY-NC-ND 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc-nd/4.0/
- Wikidata2,4,6-Tri-tert-butylanilinehttps://www.wikidata.org/wiki/Q72515485
- PubChem
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- EPA Substance Registry ServicesEPA SRS List Classificationhttps://sor.epa.gov/sor_internet/registry/substreg/LandingPage.do
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 388527142https://pubchem.ncbi.nlm.nih.gov/substance/388527142