Gold sodium thiomalate
- Gold disodium thiomalate
- Sodium aurothiomalate
- 74916-57-7
- UNII-HRS6S09A0H
- 12244-57-4
- Create:2006-03-13
- Modify:2025-01-04
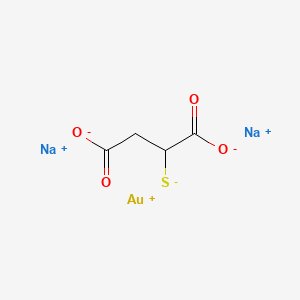
- Aurolate
- Aurothiomalate
- Aurothiomalate, Sodium
- Gold Disodium Thiomalate, Monohydrate
- Gold Sodium Thiomalate
- Gold Thiomalate
- Gold Thiomalate, Sodium
- Gold Thiomalic Acid
- Mercaptobutanedioic Acid Monogold(1+) Sodium Salt
- Miocrin
- Miocrisin
- Monogold (1+) Disodium Thiomalate
- Myochrysine
- Myocrisin
- Myocrysine
- Sodium Aurothiomalate
- Sodium Gold Thiomalate
- Sodium Thiomalate, Gold
- Sodium Thiomalatoaurate
- Tauredon
- Thiomalate, Gold
- Thiomalatoaurate, Sodium
- Gold disodium thiomalate
- Sodium aurothiomalate
- 74916-57-7
- UNII-HRS6S09A0H
- 12244-57-4
- NSC-3211
- GOLD SODIUM THIOMALATE
- HRS6S09A0H
- Butanedioic acid, mercapto-, gold(1+) disodium salt
- Butanedioic acid, 2-mercapto-, gold(1+) sodium salt (1:1:2)
- Aurolate
- NSC 147828
- disodium;gold(1+);2-sulfidobutanedioate
- Aurate(2-), ((mercapto-kappaS)butanedioato(3-)-kappaO1,kappaO4)-, disodium
- Aurate(2-), (2-(mercapto-kappaS)butanedioato(3-)-kappaO1)-, sodium (1:2)
- NSC-147828
- DTXSID80872500
- VXIHRIQNJCRFQX-UHFFFAOYSA-K
- Gold(I) disodium 2-sulfidosuccinate
- DB-041669
- GOLD(1+) DISODIUM THIOMALATE(3-)
- Gold(1+) sodium 2-sulfidobutanedioate (1:2:1)
- J-004802
- AURATE(2-), ((MERCAPTO-.KAPPA.S)BUTANEDIOATO(3-)-.KAPPA.O1,.KAPPA.O4)-, DISODIUM
- AURATE(2-), (2-(MERCAPTO-.KAPPA.S)BUTANEDIOATO(3-)-.KAPPA.O1)-, SODIUM (1:2)

H302 (100%): Harmful if swallowed [Warning Acute toxicity, oral]
H317 (100%): May cause an allergic skin reaction [Warning Sensitization, Skin]
H332 (100%): Harmful if inhaled [Warning Acute toxicity, inhalation]
P261, P264, P270, P271, P272, P280, P301+P317, P302+P352, P304+P340, P317, P321, P330, P333+P317, P362+P364, and P501
(The corresponding statement to each P-code can be found at the GHS Classification page.)
Aggregated GHS information provided per 6 reports by companies from 1 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies.
Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown.
IMAP assessments - Butanedioic acid, mercapto-, monogold(1+) sodium salt: Human health tier I assessment
IMAP assessments - Butanedioic acid, mercapto-, monogold(1+) sodium salt: Environment tier I assessment
◉ Summary of Use during Lactation
Excretion of gold into milk after gold sodium thiomalate has not been rigorously studied. Case reports indicate that gold appears in milk in small quantities and at least a little of it is absorbed because it is detectable in the infant's urine. No convincing cases of toxicity have been reported. Monitoring for possible adverse effects in the breastfed infant would seem prudent. Opinions of authors of review articles vary from recommending avoidance to allowing use. Since gold salts are rarely used any longer, an alternative is preferred.
◉ Effects in Breastfed Infants
Four infants reportedly have been breastfed during maternal gold therapy (including gold sodium thiomalate and gold aurothioglucose). Transient facial edema occurred in an 18-month-old infant, 3 months after the mother's treatment stopped. The reaction was possibly due to gold in the mother's milk ingested by the infant.
◉ Effects on Lactation and Breastmilk
Relevant published information was not found as of the revision date.
- Australian Industrial Chemicals Introduction Scheme (AICIS)Butanedioic acid, mercapto-, monogold(1+) sodium salthttps://services.industrialchemicals.gov.au/search-assessments/
- ChemIDplusGold disodium thiomalatehttps://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0074916577ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- DrugBankLICENSECreative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode)https://www.drugbank.ca/legal/terms_of_useSodium aurothiomalatehttps://www.drugbank.ca/drugs/DB09276
- EPA DSSToxGold(1+) sodium 2-sulfidobutanedioate (1:2:1)https://comptox.epa.gov/dashboard/DTXSID80872500CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-noticeSodium aurothiomalatehttps://echa.europa.eu/substance-information/-/substanceinfo/100.032.242Sodium aurothiomalate hydratehttps://echa.europa.eu/substance-information/-/substanceinfo/100.151.944Sodium aurothiomalate (EC: 235-479-7)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/46147Sodium aurothiomalate hydrate (EC: 623-247-1)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/156543
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingGOLD DISODIUM THIOMALATEhttps://gsrs.ncats.nih.gov/ginas/app/beta/substances/HRS6S09A0H
- Hazardous Substances Data Bank (HSDB)GOLD SODIUM THIOMALATEhttps://pubchem.ncbi.nlm.nih.gov/source/hsdb/7173
- ClinicalTrials.govLICENSEThe ClinicalTrials.gov data carry an international copyright outside the United States and its Territories or Possessions. Some ClinicalTrials.gov data may be subject to the copyright of third parties; you should consult these entities for any additional terms of use.https://clinicaltrials.gov/ct2/about-site/terms-conditions#Use
- NCI Thesaurus (NCIt)LICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuse
- Drugs and Lactation Database (LactMed)Gold Sodium Thiomalatehttps://www.ncbi.nlm.nih.gov/books/n/lactmed/LM129/
- EU Clinical Trials Register
- Japan Chemical Substance Dictionary (Nikkaji)
- Metabolomics WorkbenchSodium aurothiomalatehttps://www.metabolomicsworkbench.org/data/StructureData.php?RegNo=152300
- WHO Anatomical Therapeutic Chemical (ATC) ClassificationLICENSEUse of all or parts of the material requires reference to the WHO Collaborating Centre for Drug Statistics Methodology. Copying and distribution for commercial purposes is not allowed. Changing or manipulating the material is not allowed.https://www.whocc.no/copyright_disclaimer/Sodium aurothiomalatehttps://www.whocc.no/atc_ddd_index/?code=M01CB01
- Wikidata
- WikipediaSodium aurothiomalatehttps://en.wikipedia.org/wiki/Sodium_aurothiomalate
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlGold Sodium Thiomalatehttps://www.ncbi.nlm.nih.gov/mesh/68006052Antirheumatic Agentshttps://www.ncbi.nlm.nih.gov/mesh/68018501
- PubChem
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
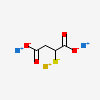

 CID 6268 (Mercaptosuccinic acid)
CID 6268 (Mercaptosuccinic acid) CID 5360545 (Sodium)
CID 5360545 (Sodium) CID 23985 (Gold)
CID 23985 (Gold)