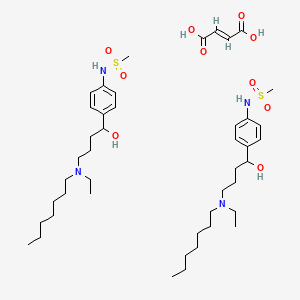
Ibutilide Fumarate
- IBUTILIDE FUMARATE
- 122647-32-9
- Corvert
- Ibutilide Fumarate [USAN]
- U-70226E
- Create:2005-06-24
- Modify:2025-01-11
- Corvert
- ibutilide
- ibutilide fumarate
- ibutilide, (+)-isomer
- ibutilide, (+-)-isomer
- ibutilide, (-)-isomer
- ibutilide, fumarate salt (2:1), (+)-isomer
- ibutilide, fumarate salt (2:1), (+-)-isomer
- N-(4-(4-(ethylheptylamino)-1-hydroxybutyl)phenyl)methanesulfonamide
- U-70226E
- U-82208E
- U-82209E
- U82208E
- U82209E
- IBUTILIDE FUMARATE
- 122647-32-9
- Corvert
- Ibutilide Fumarate [USAN]
- U-70226E
- Ibutilide hemifumarate salt
- Ibutilide hemifumarate
- UNII-9L5X4M5L6I
- 9L5X4M5L6I
- DTXSID8048652
- U 70226E
- HSDB 7926
- Methanesulfonamide, N-[4-[4-(ethylheptylamino)-1-hydroxybutyl]phenyl]-, (2E)-2-butenedioate (2:1)
- MFCD01715410
- Ibutilide (fumarate)
- DTXCID1028578
- (E)-but-2-enedioic acid;N-[4-[4-[ethyl(heptyl)amino]-1-hydroxybutyl]phenyl]methanesulfonamide
- U70226E
- 1807940-66-4
- IBUTILIDE FUMARATE (MART.)
- IBUTILIDE FUMARATE [MART.]
- IBUTILIDE FUMARATE (USP-RS)
- IBUTILIDE FUMARATE [USP-RS]
- N-(4-(4-(ethyl(heptyl)amino)-1-hydroxybutyl)phenyl)methanesulfonamide hemifumarate
- (+/-)-4'-(4-(ETHYLHEPTYLAMINO)-1-HYDROXYBUTYL)METHANESULFONANILIDE FUMARATE (2:1) (SALT)
- IBUTILIDE FUMARATE (USP MONOGRAPH)
- IBUTILIDE FUMARATE [USP MONOGRAPH]
- NCGC00181771-01
- Corvert (TN)
- Ibutilide fumarate (USP)
- (+-)-4'-(4-(Ethylheptylamino)-1-hydroxybutyl)methanesulfoanilide (E)-2-butenedioate (2:1)
- (+-)-4'-(4-(Ethylheptylamino)-1-hydroxybutyl)methanesulfonanilide fumarate (2:1) (salt)
- (+-)-N-(4-(4-(Ethylheptylamino)-1-hydroxybutyl)phenyl)methanesulfonamide (E)-butenedioate
- SCHEMBL42073
- CHEBI:5857
- IBUTILIDE FUMARATE [MI]
- CHEMBL2355456
- HMS3714I11
- HMS3884N07
- IBUTILIDE FUMARATE [VANDF]
- BCP13494
- IBUTILIDE FUMARATE [WHO-DD]
- Tox21_113006
- s2118
- AKOS015896070
- CCG-220685
- IBUTILIDE FUMARATE [ORANGE BOOK]
- AS-15378
- bis(N-(4-{4-[ethyl(heptyl)amino]-1-hydroxybutyl}phenyl)methanesulfonamide); but-2-enedioic acid
- Methanesulfonamide, N-(4-(4-(ethylheptylamino)-1-hydroxybutyl)phenyl)-, (+-)-, (E)-2-butenedioate (2:1) (salt)
- CAS-122647-32-9
- Ibutilide hemifumarate salt, >=98% (HPLC)
- SW220114-1
- A11958
- D00648
- EN300-122354
- Q27272695
- Z1541759252
- (+-)-4'-(4-(Ethylheptylamino)-1-hydroxybutyl)methanesulfonanilide Fumarate(2:1)(salt)
- bis(N-(4-(4-(ethyl(heptyl)amino)-1-hydroxybutyl)phenyl)methanesulfonamide) fumarate
- (+/-)-N-[4-[4-(Ethylheptylamino)-1-hydroxybutyl]phenyl]-methanesulfonamide hemifumarate salt
- Bis(N-(4-{4-[ethyl(heptyl)amino]-1-hydroxybutyl}phenyl)methanesulfonamide), but-2-enedioic acid
- Bis(N-(4-{4-[ethyl(heptyl)amino]-1-hydroxybutyl}phenyl)methanesulfonamide),but-2-enedioicacid
- Ibutilide fumarate, United States Pharmacopeia (USP) Reference Standard, monograph mol wt. 885.23 ([(C20H36N2O3S)2 . C4H4O4])
- METHANESULFONAMIDE, N-(4-(4-(ETHYLHEPTYLAMINO)-1-HYDROXYBUTYL)PHENYL)-, (+/-)-, (E)-2-BUTENEDIOATE (2:1) (SALT)
- N-(4-(4-(ETHYLHEPTYLAMINO)-1-HYDROXYBUTYL)PHENYL)METHANESULFONAMIDE (E)-2-BUTENEDIOATE (2:1 SALT)
385.252167 100
367.240906 48.33
144.081253 40.54
240.066956 36.64
383.234070 30.46
 Ibutilide (has active moiety)
Ibutilide (has active moiety)
P264, P270, P301+P317, P330, and P501
(The corresponding statement to each P-code can be found at the GHS Classification page.)
Aggregated GHS information provided per 39 reports by companies from 1 notifications to the ECHA C&L Inventory.
Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown.
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=PCIOHQNIRPWFMV-WXXKFALUSA-N
- ChEBIIbutilide fumaratehttps://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:5857
- NCI Thesaurus (NCIt)LICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuseNCI Thesaurushttps://ncit.nci.nih.gov
- Open TargetsLICENSEDatasets generated by the Open Targets Platform are freely available for download.https://platform-docs.opentargets.org/licenceIBUTILIDE FUMARATEhttps://platform.opentargets.org/drug/CHEMBL2355456
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.htmlChEMBL Protein Target Treehttps://www.ebi.ac.uk/chembl/g/#browse/targets
- ChemIDplusIbutilide Fumarate [USAN]https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0122647329ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- EPA DSSToxIbutilide fumaratehttps://comptox.epa.gov/dashboard/DTXSID8048652CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-noticeIbutilide Hemifumaratehttps://echa.europa.eu/substance-information/-/substanceinfo/100.213.279Ibutilide Hemifumarate (EC: 687-381-2)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/218376
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingIBUTILIDE FUMARATEhttps://gsrs.ncats.nih.gov/ginas/app/beta/substances/9L5X4M5L6I
- ClinicalTrials.govLICENSEThe ClinicalTrials.gov data carry an international copyright outside the United States and its Territories or Possessions. Some ClinicalTrials.gov data may be subject to the copyright of third parties; you should consult these entities for any additional terms of use.https://clinicaltrials.gov/ct2/about-site/terms-conditions#Use
- DailyMed
- Drugs@FDALICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingIBUTILIDE FUMARATEhttps://www.accessdata.fda.gov/scripts/cder/daf/
- EU Clinical Trials Register
- FDA Orange BookLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.htmlAnatomical Therapeutic Chemical (ATC) classificationhttp://www.genome.jp/kegg-bin/get_htext?br08303.kegTarget-based classification of drugshttp://www.genome.jp/kegg-bin/get_htext?br08310.keg
- MassBank of North America (MoNA)LICENSEThe content of the MoNA database is licensed under CC BY 4.0.https://mona.fiehnlab.ucdavis.edu/documentation/license
- National Drug Code (NDC) DirectoryLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- NLM RxNorm TerminologyLICENSEThe RxNorm Terminology is created by the National Library of Medicine (NLM) and is in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from NLM. Credit to the U.S. National Library of Medicine as the source is appreciated but not required. The full RxNorm dataset requires a free license.https://www.nlm.nih.gov/research/umls/rxnorm/docs/termsofservice.htmlibutilide fumaratehttps://rxnav.nlm.nih.gov/id/rxnorm/51256
- Springer Nature
- Wikidataibutilide fumaratehttps://www.wikidata.org/wiki/Q27272695
- PubChem
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlAnti-Arrhythmia Agentshttps://www.ncbi.nlm.nih.gov/mesh/68000889
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 389003483https://pubchem.ncbi.nlm.nih.gov/substance/389003483

 CID 444972 (Fumaric Acid)
CID 444972 (Fumaric Acid)