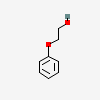
Phenoxyethanol
- C8H10O2
- C6H5OC2H4OH
- 2-PHENOXYETHANOL
- Phenoxyethanol
- 122-99-6
- Ethylene glycol monophenyl ether
- Phenyl cellosolve
- Create:2005-03-26
- Modify:2025-01-04
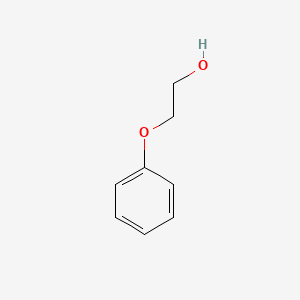
C8H10O2
C6H5OC2H4OH
- 2-phenoxyethanol
- Emuclens
- Erisept
- ethylene glycol monophenyl ether
- phenoxethol
- phenoxyethanol
- 2-PHENOXYETHANOL
- Phenoxyethanol
- 122-99-6
- Ethylene glycol monophenyl ether
- Phenyl cellosolve
- Phenoxethol
- Ethanol, 2-phenoxy-
- Phenoxytol
- 2-Phenoxyethan-1-Ol
- Ethylene glycol phenyl ether
- 1-Hydroxy-2-phenoxyethane
- Phenoxetol
- Phenoxyethyl alcohol
- Rose ether
- Phenylmonoglycol ether
- Arosol
- Fenyl-cellosolve
- 2-Fenoxyethanol
- Dowanol EP
- 2-Phenoxyethyl alcohol
- Glycol monophenyl ether
- 2-Hydroxyethyl phenyl ether
- Fenylcelosolv
- Phenylglycol
- Dowanol EPH
- 2-Phenoxy-ethanol
- Emery 6705
- Emeressence 1160
- EGMPE
- beta-Hydroxyethyl phenyl ether
- MFCD00002857
- Plastiazan-41
- NSC 1864
- PHE-G
- .beta.-Hydroxyethyl phenyl ether
- HSDB 5595
- UNII-HIE492ZZ3T
- NSC-1864
- 9004-78-8
- EINECS 204-589-7
- HIE492ZZ3T
- BRN 1364011
- CCRIS 9481
- Phenoxyethanol [NF]
- Ethylene glycol-monophenyl ether
- AI3-00752()C
- DTXSID9021976
- CHEBI:64275
- PHE-S
- .beta.-Phenoxyethyl alcohol
- DTXCID401976
- 2-Phenoxyethyl--d4 Alcohol
- FEMA NO. 4620
- EC 204-589-7
- 4-06-00-00571 (Beilstein Handbook Reference)
- Phenoxyethanol (NF)
- NCGC00090731-01
- NCGC00090731-05
- PHENOXYETHANOL (II)
- PHENOXYETHANOL [II]
- PHG
- PHENOXYETHANOL (MART.)
- PHENOXYETHANOL [MART.]
- PHENOXYETHANOL (USP-RS)
- PHENOXYETHANOL [USP-RS]
- Fenylcelosolv [Czech]
- 2-Fenoxyethanol [Czech]
- Fenyl-cellosolve [Czech]
- PHENOXYETHANOL (EP MONOGRAPH)
- PHENOXYETHANOL [EP MONOGRAPH]
- Plastiazan-41 [Russian]
- beta-Phenoxyethanol
- CAS-122-99-6
- .beta.-Phenoxyethanol
- phenylcellosolve
- Phenoxyethanolum
- Dalpad A
- 2-phenyloxyethanol
- Newpol EFP
- 2-phenoxy ethanol
- 2-(phenoxy)ethanol
- 2-phenoxy-1-ethanol
- CPAP WIPES
- VAXOL PURI
- beta-phenoxyethylalcohol
- starbld0047047
- 2-Phenoxyethanol, 9CI
- 2-Phenoxyethanol, 99%
- Phenoxyethanol (Standard)
- WLN: Q2OR
- SCHEMBL15708
- 2-Phenoxyethanol, >=99%
- PHENOXYETHANOL [HSDB]
- MLS002174254
- ethyleneglycol monophenyl ether
- Euxyl K 400 (Salt/Mix)
- ProClin? 8020 preservatives
- 2-PHENOXYETHANOL [MI]
- Fungal Terminator (veterinary)
- PHENOXYETHANOL [WHO-DD]
- CHEMBL1229846
- HY-B1729R
- NSC1864
- HMS2268A20
- HY-B1729
- STR04582
- Tox21_111002
- Tox21_113532
- Tox21_202111
- Tox21_300842
- BBL027410
- STK802556
- 2-Phenoxyethanol, analytical standard
- Fungal Terminator [veterinary] (TN)
- AKOS000118741
- Tox21_111002_1
- 1ST2538
- DB11304
- NCGC00090731-02
- NCGC00090731-03
- NCGC00090731-04
- NCGC00090731-06
- NCGC00090731-07
- NCGC00090731-08
- NCGC00254745-01
- NCGC00259660-01
- 56257-90-0
- DA-76810
- Ethylene glycol monophenyl ether, >=90%
- SMR000112131
- ETHANOL,2-PHENOXY MFC8 H10 O2
- CS-0013737
- NS00002984
- P0115
- P1953
- EN300-19339
- 2-Phenoxyethanol, tested according to Ph.Eur.
- D08359
- G74506
- SBI-0653920.0001
- Q418038
- SR-01000838345
- J-510235
- SR-01000838345-2
- F1905-6997
- Z104473570
- Ethylene glycol monophenyl ether, SAJ first grade, >=95.0%
- Phenoxyethanol, European Pharmacopoeia (EP) Reference Standard
- Phenoxyethanol, United States Pharmacopeia (USP) Reference Standard
- InChI=1/C8H10O2/c9-6-7-10-8-4-2-1-3-5-8/h1-5,9H,6-7H
- 2-Phenoxyethanol, Pharmaceutical Secondary Standard; Certified Reference Material
94.0 99.99
138.0 27.93
77.0 23.31
95.0 12.84
28.0 10.68
94.0 99.99
138.0 41.80
77.0 29.40
45.0 27.70
66.0 16.40
121.07 43.17
91.05 25.79
103.08 6.02
93.07 5.43
85.06 4.34
121.06 38.67
91.05 25.08
93.07 6.94
103.08 6.07
85.06 5.20
94 999
138 279
77 233
95 128
28 107
- Alcohol; benzalkonium chloride; methylisothiazolinone; phenoxyethanol; propylene glycol (component of)
- Benzoic acid; butylene glycol; dehydroacetic acid; edetic acid; fragrance 13576; glycerin; phenoxyethanol; polysorbate 20; propanediol; proteoglycan 4; water; xanthan gum (component of)
- BENZOIC ACID; BENZYL ALCOHOL; DEHYDROACETIC ACID; EDETIC ACID; FRAGRANCE 13576; GLYCERIN; HYDROXYCITRONELLAL; ISOMETHYL-alpha-IONONE; PEG-8 CAPRYLIC/CAPRIC GLYCERIDES; PHENOXYETHANOL; PROPANEDIOL; SORBIC ACID; WATER (component of)
- Alcohol; ammonia; ascorbyl glucoside; ethylhexylglycerin; glycolic acid; hexylresorcinol; hydroxyethyl cellulose, unspecified; isopropyl alcohol; kojic acid; lactic acid; phenoxyethanol; propylene glycol; salicylic acid; water (component of)
- beta-CITRONELLOL, (R)-; ALCOHOL; BUTYLATED HYDROXYTOLUENE; C12-20 ACID PEG-8 ESTER; CETEARYL ETHYLHEXANOATE; ETHYLHEXYLGLYCERIN; FRAGRANCE 13576; GLYCERIN; GLYCERYL MONOSTEARATE; GRAPE SEED OIL; HYDROXYACETOPHENONE; ISOMETHYL-alpha-IONONE; LINALOOL, (+/-)-; PALMITIC ACID; PERLITE; PHENOXYETHANOL; PROPYLENE GLYCOL; STARCH, RICE; STEARIC ACID; TITANIUM DIOXIDE; TROLAMINE; WATER (component of)
- alpha-HEXYLCINNAMALDEHYDE; beta-CITRONELLOL, (R)-; AVOBENZONE; BENZOIC ACID; BUTYLATED HYDROXYTOLUENE; CENTAUREA CYANUS FLOWER; CETOSTEARYL ALCOHOL; CETYL ALCOHOL; COCO-GLYCERIDES; DEHYDROACETIC ACID; FRAGRANCE 13576; GLYCERIN; HYDROLYSED MARINE COLLAGEN (ENZYMATIC; 2000 MW); HYDROLYZED BOVINE ELASTIN (BASE; 1000 MW); ISOMETHYL-alpha-IONONE; LIMONENE, (+)-; MEDIUM-CHAIN TRIGLYCERIDES; OCTINOXATE; PHENOXYETHANOL; POTASSIUM PHOSPHATE, UNSPECIFIED FORM; VIOLA ODORATA; WATER (component of)
- Ascorbic acid; ascorbyl palmitate; avobenzone; bemotrizinol; butylated hydroxytoluene; butylene glycol; C20-22 alcohols; carbomer copolymer type A (allyl pentaerythritol crosslinked); carbomer homopolymer, unspecified type; chlorphenesin; dimethicone; fragrance 13576; glycerin; linoleic acid; linolenic acid; octinoxate; octisalate; octocrylene; phenoxyethanol; poly(methyl methacrylate; 450000 MW); polyethylene glycol 400; propylene glycol; silicon dioxide; titanium dioxide; tocopherol; tromethamine; water (component of)
- alpha-TOCOPHEROL; ANHYDROUS CITRIC ACID; ASCORBIC ACID; ASCORBYL PALMITATE; AVOBENZONE; BEMOTRIZINOL; BUTYLATED HYDROXYTOLUENE; BUTYLENE GLYCOL; C20-22 ALCOHOLS; CARBOMER COPOLYMER TYPE A (ALLYL PENTAERYTHRITOL CROSSLINKED); CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE; CHLORPHENESIN; DIMETHICONE; FRAGRANCE 13576; GLYCERIN; LINOLEIC ACID; LINOLENIC ACID; OCTINOXATE; OCTISALATE; OCTOCRYLENE; PHENOXYETHANOL; POLY(METHYL METHACRYLATE; 450000 MW); POLYETHYLENE GLYCOL 400; PROPYLENE GLYCOL; SILICON DIOXIDE; TITANIUM DIOXIDE; TROMETHAMINE; WATER (component of)
- Cytoplasm
- Extracellular
Metal Degreasing [Category: Clean]
Using Disinfectants or Biocides [Category: Clean]
Textiles (Printing, Dyeing, or Finishing) [Category: Industry]
Use (kg; approx.) in Germany (2009): >25000
Consumption (g per capita; approx.) in Germany (2009): 0.305
Calculated removal (%): 92.1
 Yellow triangle - The chemical has met Safer Choice Criteria for its functional ingredient-class, but has some hazard profile issues
Yellow triangle - The chemical has met Safer Choice Criteria for its functional ingredient-class, but has some hazard profile issues- Solvents (which become part of product formulation or mixture)
- Other (specify)
- Surface modifier
- Anti-adhesive/cohesive
- Solvent
- Not Known or Reasonably Ascertainable
- Preservative
- Paint additives and coating additives not described by other categories
- Surfactant (surface active agent)
- Plasticizer
- Odor agents
- Cleaning agent
- Preservative
- Paint additives and coating additives not described by other categories
- Cleaning agent
- Plasticizer
- Odor agents
- Laboratory chemicals
- Other (specify)
- Not Known or Reasonably Ascertainable
- Solvent
Cosmetics product ingredient: 2-Phenoxyethanol
Reason for Listing: Identified as a Toxic Air Contaminant by the State Board as defined in Health and Safety Code section 39655.
Potential Health Impacts: Respiratory Toxicity
Product count: 1756
Information on 734 consumer products that contain 2-Phenoxyethanol in the following categories is provided:
• Auto Products
• Commercial / Institutional
• Home Maintenance
• Inside the Home
• Personal Care
• Pet Care
Information on 13 consumer products that contain Poly(oxy-1,2-ethanediyl), alpha-phenyl-omega-hydroxy- in the following categories is provided:
• Auto Products
• Inside the Home
2019: 20,000,000 lb - <100,000,000 lb
2018: 20,000,000 lb - <100,000,000 lb
2017: 20,000,000 lb - <100,000,000 lb
2016: 20,000,000 lb - <100,000,000 lb
- Petroleum Lubricating Oil and Grease Manufacturing
- Wholesale and Retail Trade
- Miscellaneous Manufacturing
- All Other Basic Organic Chemical Manufacturing
- Paint and Coating Manufacturing
- Transportation Equipment Manufacturing
- Adhesive Manufacturing
- Other (requires additional information)
- Printing Ink Manufacturing
- Plastics Product Manufacturing
- Printing and Related Support Activities
- Non-metallic Mineral Product Manufacturing (includes clay, glass, cement, concrete, lime, gypsum, and other non-metallic mineral product manufacturing)
- Not Known or Reasonably Ascertainable
- Soap, Cleaning Compound, and Toilet Preparation Manufacturing


H302 (99.8%): Harmful if swallowed [Warning Acute toxicity, oral]
H318 (11.5%): Causes serious eye damage [Danger Serious eye damage/eye irritation]
H319 (88.5%): Causes serious eye irritation [Warning Serious eye damage/eye irritation]
H335 (11.5%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation]
P261, P264, P264+P265, P270, P271, P280, P301+P317, P304+P340, P305+P351+P338, P305+P354+P338, P317, P319, P330, P337+P317, P403+P233, P405, and P501
(The corresponding statement to each P-code can be found at the GHS Classification page.)
Aggregated GHS information provided per 4266 reports by companies from 37 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies.
Reported as not meeting GHS hazard criteria per 1 of 4266 reports by companies. For more detailed information, please visit ECHA C&L website.
There are 36 notifications provided by 4265 of 4266 reports by companies with hazard statement code(s).
Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown.
Acute Tox. 4 (99.8%)
Eye Dam. 1 (11.5%)
Eye Irrit. 2 (88.5%)
STOT SE 3 (11.5%)
Acute Tox. 4 (41.7%)
Skin Irrit. 2 (57.8%)
Eye Dam. 1 (14.8%)
Eye Irrit. 2 (65.2%)

Chemical: Phenoxyethanol

EYES: First check the victim for contact lenses and remove if present. Flush victim's eyes with water or normal saline solution for 20 to 30 minutes while simultaneously calling a hospital or poison control center. Do not put any ointments, oils, or medication in the victim's eyes without specific instructions from a physician. IMMEDIATELY transport the victim after flushing eyes to a hospital even if no symptoms (such as redness or irritation) develop.
SKIN: IMMEDIATELY flood affected skin with water while removing and isolating all contaminated clothing. Gently wash all affected skin areas thoroughly with soap and water. If symptoms such as redness or irritation develop, IMMEDIATELY call a physician and be prepared to transport the victim to a hospital for treatment.
INHALATION: IMMEDIATELY leave the contaminated area; take deep breaths of fresh air. If symptoms (such as wheezing, coughing, shortness of breath, or burning in the mouth, throat, or chest) develop, call a physician and be prepared to transport the victim to a hospital. Provide proper respiratory protection to rescuers entering an unknown atmosphere. Whenever possible, Self-Contained Breathing Apparatus (SCBA) should be used; if not available, use a level of protection greater than or equal to that advised under Protective Clothing.
INGESTION: DO NOT INDUCE VOMITING. If the victim is conscious and not convulsing, give 1 or 2 glasses of water to dilute the chemical and IMMEDIATELY call a hospital or poison control center. Be prepared to transport the victim to a hospital if advised by a physician. If the victim is convulsing or unconscious, do not give anything by mouth, ensure that the victim's airway is open and lay the victim on his/her side with the head lower than the body. DO NOT INDUCE VOMITING. IMMEDIATELY transport the victim to a hospital. (NTP, 1992)
SMALL SPILLS AND LEAKAGE: If you should spill this chemical, use absorbent paper to pick up all liquid spill material. Seal the absorbent paper, as well as any of your clothing which may be contaminated, in a vapor-tight plastic bag for eventual disposal. Wash any surfaces you may have contaminated with a soap and water solution. Do not reenter the contaminated area until the Safety Officer (or other responsible person) has verified that the area has been properly cleaned.
STORAGE PRECAUTIONS: You should store this material under ambient temperatures, and keep it away from oxidizing materials. (NTP, 1992)
Alcohols and Polyols
Ethers
Hydrocarbons, Aromatic
Hazard Traits - Respiratory Toxicity
Authoritative List - CA TACs
Report - if used as a fragrance or flavor ingredient
Neurotoxin - Acute solvent syndrome
Occupational hepatotoxin - Secondary hepatotoxins: the potential for toxic effect in the occupational setting is based on cases of poisoning by human ingestion or animal experimentation.
Nephrotoxin - The chemical is potentially toxic to the kidneys in the occupational setting.
Reproductive Toxin - A chemical that is toxic to the reproductive system, including defects in the progeny and injury to male or female reproductive function. Reproductive toxicity includes developmental effects. See Guidelines for Reproductive Toxicity Risk Assessment.
Skin Sensitizer - An agent that can induce an allergic reaction in the skin.
Contact dermatitis, allergic [Category: Skin Disease]
Solvents, acute toxic effect [Category: Acute Poisoning]
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=QCDWFXQBSFUVSP-UHFFFAOYSA-N
- Australian Industrial Chemicals Introduction Scheme (AICIS)Ethanol, 2-phenoxy-https://services.industrialchemicals.gov.au/search-assessments/Poly(oxy-1,2-ethanediyl), .alpha.-phenyl-.omega.-hydroxy-https://services.industrialchemicals.gov.au/search-inventory/
- CAMEO ChemicalsLICENSECAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data.https://cameochemicals.noaa.gov/help/reference/terms_and_conditions.htm?d_f=falseETHYLENE GLYCOL PHENYL ETHERhttps://cameochemicals.noaa.gov/chemical/17818CAMEO Chemical Reactivity Classificationhttps://cameochemicals.noaa.gov/browse/react
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/Ethoxylated phenolhttps://commonchemistry.cas.org/detail?cas_rn=9004-78-8Phenoxyethanolhttps://commonchemistry.cas.org/detail?cas_rn=122-99-6
- ChemIDplusPhenoxyethanol [NF]https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000122996ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- DrugBankLICENSECreative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode)https://www.drugbank.ca/legal/terms_of_usePhenoxyethanolhttps://www.drugbank.ca/drugs/DB11304
- DTP/NCILICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuse
- EPA Chemical Data Reporting (CDR)LICENSEThe U.S. Government retains a nonexclusive, royalty-free license to publish or reproduce these documents, or allow others to do so, for U.S. Government purposes. These documents may be freely distributed and used for non-commercial, scientific and educational purposes.https://www.epa.gov/web-policies-and-procedures/epa-disclaimers#copyrightEthanol, 2-phenoxy-https://www.epa.gov/chemical-data-reporting
- EPA Chemicals under the TSCAEthanol, 2-phenoxy-https://www.epa.gov/chemicals-under-tscaEPA TSCA Classificationhttps://www.epa.gov/tsca-inventory
- EPA DSSTox2-Phenoxyethanolhttps://comptox.epa.gov/dashboard/DTXSID9021976CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-notice2-phenoxyethanolhttps://chem.echa.europa.eu/100.004.173Phenol, ethoxylatedhttps://chem.echa.europa.eu/100.105.5232-phenoxyethanol (EC: 204-589-7)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/57792Phenol, ethoxylated (EC: 500-013-6)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/76964
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- Hazardous Substances Data Bank (HSDB)2-PHENOXYETHANOLhttps://pubchem.ncbi.nlm.nih.gov/source/hsdb/5595
- Human Metabolome Database (HMDB)LICENSEHMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications.http://www.hmdb.ca/citing2-Phenoxyethanolhttp://www.hmdb.ca/metabolites/HMDB0041607HMDB0041607_cms_102229https://hmdb.ca/metabolites/HMDB0041607#spectra
- ILO-WHO International Chemical Safety Cards (ICSCs)ETHYLENE GLYCOL MONOPHENYL ETHERhttps://www.ilo.org/dyn/icsc/showcard.display?p_version=2&p_card_id=0538
- International Fragrance Association (IFRA)LICENSE(c) The International Fragrance Association, 2007-2021. All rights reserved.https://ifrafragrance.org/links/copyright
- New Zealand Environmental Protection Authority (EPA)LICENSEThis work is licensed under the Creative Commons Attribution-ShareAlike 4.0 International licence.https://www.epa.govt.nz/about-this-site/general-copyright-statement/
- California Safe Cosmetics Program (CSCP) Product Database2-Phenoxyethanolhttps://cscpsearch.cdph.ca.gov/search/detailresult/4237
- Consumer Product Information Database (CPID)LICENSECopyright (c) 2024 DeLima Associates. All rights reserved. Unless otherwise indicated, all materials from CPID are copyrighted by DeLima Associates. No part of these materials, either text or image may be used for any purpose other than for personal use. Therefore, reproduction, modification, storage in a retrieval system or retransmission, in any form or by any means, electronic, mechanical or otherwise, for reasons other than personal use, is strictly prohibited without prior written permission.https://www.whatsinproducts.com/contents/view/1/6Poly(oxy-1,2-ethanediyl), alpha-phenyl-omega-hydroxy-https://www.whatsinproducts.com/chemicals/view/1/6355/009004-78-8Consumer Products Category Classificationhttps://www.whatsinproducts.com/
- Cosmetic Ingredient Review (CIR)
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/2-PHENOXYETHANOLNORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- Haz-Map, Information on Hazardous Chemicals and Occupational DiseasesLICENSECopyright (c) 2022 Haz-Map(R). All rights reserved. Unless otherwise indicated, all materials from Haz-Map are copyrighted by Haz-Map(R). No part of these materials, either text or image may be used for any purpose other than for personal use. Therefore, reproduction, modification, storage in a retrieval system or retransmission, in any form or by any means, electronic, mechanical or otherwise, for reasons other than personal use, is strictly prohibited without prior written permission.https://haz-map.com/AboutEthylene glycol monophenyl etherhttps://haz-map.com/Agents/1901
- ChEBI2-phenoxyethanolhttps://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:64275
- LOTUS - the natural products occurrence databaseLICENSEThe code for LOTUS is released under the GNU General Public License v3.0.https://lotus.nprod.net/2-Phenoxyethanolhttps://www.wikidata.org/wiki/Q418038LOTUS Treehttps://lotus.naturalproducts.net/
- Open TargetsLICENSEDatasets generated by the Open Targets Platform are freely available for download.https://platform-docs.opentargets.org/licencePHENOXYETHANOLhttps://platform.opentargets.org/drug/CHEMBL1229846
- Toxin and Toxin Target Database (T3DB)LICENSET3DB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (T3DB) and the original publication.http://www.t3db.ca/downloads2-Phenoxyethanolhttp://www.t3db.ca/toxins/T3D3900
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.html
- Comparative Toxicogenomics Database (CTD)LICENSEIt is to be used only for research and educational purposes. Any reproduction or use for commercial purpose is prohibited without the prior express written permission of NC State University.http://ctdbase.org/about/legal.jspphenoxyethanolhttps://ctdbase.org/detail.go?type=chem&acc=C005398
- Drug Gene Interaction database (DGIdb)LICENSEThe data used in DGIdb is all open access and where possible made available as raw data dumps in the downloads section.http://www.dgidb.org/downloadsPHENOXYETHANOLhttps://www.dgidb.org/drugs/rxcui:89552
- EPA Chemical and Products Database (CPDat)EPA CPDat Classificationhttps://www.epa.gov/chemical-research/chemical-and-products-database-cpdat
- Crystallography Open Database (COD)LICENSEAll data in the COD and the database itself are dedicated to the public domain and licensed under the CC0 License. Users of the data should acknowledge the original authors of the structural data.https://creativecommons.org/publicdomain/zero/1.0/
- DailyMed
- EPA Safer ChoicePhenoxyethanolhttps://www.epa.gov/saferchoice/safer-ingredientsEPA Safer Chemical Ingredients Classificationhttps://www.epa.gov/saferchoice
- EU Clinical Trials Register
- Hazardous Chemical Information System (HCIS), Safe Work Australia
- NITE-CMC2-Phenoxyethanol - FY2013 (Revised classification)https://www.chem-info.nite.go.jp/chem/english/ghs/13-mhlw-2012e.html2-phenoxyethanol - FY2008 (New/original classication)https://www.chem-info.nite.go.jp/chem/english/ghs/08-mhlw-0182e.html2-Phenoxyethanol - FY2022 (Revised classification)https://www.chem-info.nite.go.jp/chem/english/ghs/22-jniosh-2085e.html
- Regulation (EC) No 1272/2008 of the European Parliament and of the CouncilLICENSEThe copyright for the editorial content of this source, the summaries of EU legislation and the consolidated texts, which is owned by the EU, is licensed under the Creative Commons Attribution 4.0 International licence.https://eur-lex.europa.eu/content/legal-notice/legal-notice.html2-phenoxyethanolhttps://eur-lex.europa.eu/eli/reg/2008/1272/oj
- FDA Substances Added to FoodLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingETHYLENE GLYCOL MONOPHENYL ETHERhttps://www.hfpappexternal.fda.gov/scripts/fdcc/index.cfm?set=FoodSubstances&id=ETHYLENEGLYCOLMONOPHENYLETHER
- Flavor and Extract Manufacturers Association (FEMA)2-PHENOXYETHANOLhttps://www.femaflavor.org/flavor-library/2-phenoxyethanol
- IUPAC Digitized pKa Datasetethanol, 2-phenoxy-https://github.com/IUPAC/Dissociation-Constants
- NMRShiftDB
- MassBank Europe
- SpectraBase2-Phenoxyethanolhttps://spectrabase.com/spectrum/DrWOkoD52xrPHENOXYETHANOLhttps://spectrabase.com/spectrum/5dFPD4iqe6yETHANOL, 2-PHENOXY-https://spectrabase.com/spectrum/HRQTIzFV8cF2-Phenoxyethanolhttps://spectrabase.com/spectrum/FgIR1zH3a3r2-Phenoxyethanolhttps://spectrabase.com/spectrum/KA2XqGmPy0a2-Phenoxyethanolhttps://spectrabase.com/spectrum/F0mwtRtP92G2-Phenoxyethanolhttps://spectrabase.com/spectrum/Kp5gx23ccGv2-Phenoxyethanolhttps://spectrabase.com/spectrum/Igfcg6Imxth2-Phenoxyethanolhttps://spectrabase.com/spectrum/Lt7nZNBTV2m2-Phenoxyethanolhttps://spectrabase.com/spectrum/BaJJwb3RnPc2-Phenoxyethanolhttps://spectrabase.com/spectrum/21GP5399OXv2-Phenoxyethanolhttps://spectrabase.com/spectrum/5ql3YCUovwb2-Phenoxyethanolhttps://spectrabase.com/spectrum/LIE8MH4hpMi2-Phenoxyethanol, phenylglycolhttps://spectrabase.com/spectrum/FXFpX3fAtiDEthanol, 2-phenoxy-https://spectrabase.com/spectrum/Ce7UVIOKF16Ethanol, 2-phenoxy-https://spectrabase.com/spectrum/VoJ9dyiNxd
- MassBank of North America (MoNA)LICENSEThe content of the MoNA database is licensed under CC BY 4.0.https://mona.fiehnlab.ucdavis.edu/documentation/license
- NIST Mass Spectrometry Data CenterLICENSEData covered by the Standard Reference Data Act of 1968 as amended.https://www.nist.gov/srd/public-lawEthanol, 2-phenoxy-http://www.nist.gov/srd/nist1a.cfm
- Japan Chemical Substance Dictionary (Nikkaji)
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.html
- KNApSAcK Species-Metabolite Databasebeta-Hydroxyethyl phenyl etherhttp://www.knapsackfamily.com/knapsack_core/info.php?sname=C_ID&word=C00055732
- Natural Product Activity and Species Source (NPASS)2-Phenoxyethanolhttps://bidd.group/NPASS/compound.php?compoundID=NPC318429
- Metabolomics Workbench
- National Drug Code (NDC) DirectoryLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- NCI Thesaurus (NCIt)LICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuseNCI Thesaurushttps://ncit.nci.nih.gov
- NLM RxNorm TerminologyLICENSEThe RxNorm Terminology is created by the National Library of Medicine (NLM) and is in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from NLM. Credit to the U.S. National Library of Medicine as the source is appreciated but not required. The full RxNorm dataset requires a free license.https://www.nlm.nih.gov/research/umls/rxnorm/docs/termsofservice.htmlphenoxyethanolhttps://rxnav.nlm.nih.gov/id/rxnorm/89552
- Protein Data Bank in Europe (PDBe)
- RCSB Protein Data Bank (RCSB PDB)LICENSEData files contained in the PDB archive (ftp://ftp.wwpdb.org) are free of all copyright restrictions and made fully and freely available for both non-commercial and commercial use. Users of the data should attribute the original authors of that structural data.https://www.rcsb.org/pages/policies
- Springer Nature
- SpringerMaterials
- Thieme ChemistryLICENSEThe Thieme Chemistry contribution within PubChem is provided under a CC-BY-NC-ND 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc-nd/4.0/
- Wikidataphenoxyethanolhttps://www.wikidata.org/wiki/Q418038
- WikipediaBrofarominehttps://en.wikipedia.org/wiki/BrofarominePhenoxyethanolhttps://en.wikipedia.org/wiki/Phenoxyethanol
- Wiley
- PubChem
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlphenoxyethanolhttps://www.ncbi.nlm.nih.gov/mesh/67005398Anestheticshttps://www.ncbi.nlm.nih.gov/mesh/68000777Anti-Infective Agents, Localhttps://www.ncbi.nlm.nih.gov/mesh/68000891
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- EPA Substance Registry ServicesEPA SRS List Classificationhttps://sor.epa.gov/sor_internet/registry/substreg/LandingPage.do
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 403029838https://pubchem.ncbi.nlm.nih.gov/substance/403029838