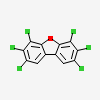
2,3,4,6,7,8-Hexachlorodibenzofuran
- 2,3,4,6,7,8-HEXACHLORODIBENZOFURAN
- 60851-34-5
- 2,3,4,6,7,8-HxCDF
- Dibenzofuran, 2,3,4,6,7,8-hexachloro-
- UNII-IMR03169ES
- Create:2005-03-27
- Modify:2025-01-18
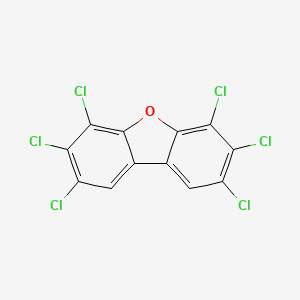
- 2,3,4,6,7,8-HEXACHLORODIBENZOFURAN
- 60851-34-5
- 2,3,4,6,7,8-HxCDF
- Dibenzofuran, 2,3,4,6,7,8-hexachloro-
- UNII-IMR03169ES
- IMR03169ES
- PCDF 130
- CHEMBL343049
- DTXSID3052276
- CHEBI:81512
- 2,3,4,6,7,8-Hexachlorodibenzo(b,d)furan
- 2,3,4,6,7,8-Hexachlorodibenzo[b,d]furan
- F 130
- 4,5,6,10,11,12-hexachloro-8-oxatricyclo(7.4.0.02,7)trideca-1(13),2,4,6,9,11-hexaene
- 4,5,6,10,11,12-hexachloro-8-oxatricyclo[7.4.0.02,7]trideca-1(13),2,4,6,9,11-hexaene
- SCHEMBL2278973
- DTXCID3030848
- BDBM50408302
- 2,3,4,6,7,8-Hexa-chlorodibenzofuran
- 2,3,4,6,7,8-Hexachloro-dibenzofuran
- CS-0832761
- NS00098496
- 2,3,4,6,7,8-Hexachlorodibenzo[b,d]furan #
- C18110
- Q27155434

H301 (100%): Toxic if swallowed [Danger Acute toxicity, oral]
H319 (100%): Causes serious eye irritation [Warning Serious eye damage/eye irritation]
H413 (100%): May cause long lasting harmful effects to aquatic life [Hazardous to the aquatic environment, long-term hazard]
P264, P264+P265, P270, P273, P280, P301+P316, P305+P351+P338, P321, P330, P337+P317, P405, and P501
(The corresponding statement to each P-code can be found at the GHS Classification page.)
Aggregated GHS information provided per 39 reports by companies from 1 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies.
Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown.
Acute Tox. 3 (100%)
Eye Irrit. 2 (100%)
Aquatic Chronic 4 (100%)
Hazard Traits - Bioaccumulation; Carcinogenicity; Dermatotoxicity; Developmental Toxicity; Endocrine Toxicity; Environmental Persistence; Environmental tox; Hematotoxicity; Hepatotoxicity and Digestive System Toxicity; Reproductive Toxicity
Authoritative List - CA TACs; CWA 303(d); OEHHA RELs; WA PBTs
Report - if used as a fragrance or flavor ingredient
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=XTAHLACQOVXINQ-UHFFFAOYSA-N
- California Safe Cosmetics Program (CSCP) Product Database2,3,4,6,7,8-Hexachlorodibenzo-p-furan (HxCDF)https://www.cdph.ca.gov/Programs/CCDPHP/DEODC/OHB/CSCP/Pages/About-CSCP.aspx
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/2,3,4,6,7,8-Hexachlorodibenzofuranhttps://commonchemistry.cas.org/detail?cas_rn=60851-34-5
- ChemIDplus2,3,4,6,7,8-Hexachlorodibenzofuranhttps://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0060851345ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- EPA DSSTox2,3,4,6,7,8-Hexachlorodibenzofuranhttps://comptox.epa.gov/dashboard/DTXSID3052276CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-notice2,3,4,6,7,8-Hexachlorodibenzofuranhttps://echa.europa.eu/substance-information/-/substanceinfo/100.223.0472,3,4,6,7,8-Hexachlorodibenzofuran (EC: 694-831-1)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/228901
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking2,3,4,6,7,8-HEXACHLORODIBENZOFURANhttps://gsrs.ncats.nih.gov/ginas/app/beta/substances/IMR03169ES
- Risk Assessment Information System (RAIS)LICENSEThis work has been sponsored by the U.S. Department of Energy (DOE), Office of Environmental Management, Oak Ridge Operations (ORO) Office through a joint collaboration between United Cleanup Oak Ridge LLC (UCOR), Oak Ridge National Laboratory (ORNL), and The University of Tennessee, Ecology and Evolutionary Biology, The Institute for Environmental Modeling (TIEM). All rights reserved.https://rais.ornl.gov/HxCDF, 2,3,4,6,7,8-https://rais.ornl.gov/cgi-bin/tools/TOX_search
- ChEBI2,3,4,6,7,8-Hexachlorodibenzofuranhttps://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:81512
- Toxin and Toxin Target Database (T3DB)LICENSET3DB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (T3DB) and the original publication.http://www.t3db.ca/downloads2,3,4,6,7,8-Hexachlorodibenzofuranhttp://www.t3db.ca/toxins/T3D2229
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.htmlChEMBL Protein Target Treehttps://www.ebi.ac.uk/chembl/g/#browse/targets
- Human Metabolome Database (HMDB)LICENSEHMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications.http://www.hmdb.ca/citing2,3,4,6,7,8-Hexachlorodibenzofuranhttp://www.hmdb.ca/metabolites/HMDB0245433
- Japan Chemical Substance Dictionary (Nikkaji)
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.htmlEndocrine disrupting compoundshttp://www.genome.jp/kegg-bin/get_htext?br08006.keg
- NIST Mass Spectrometry Data CenterLICENSEData covered by the Standard Reference Data Act of 1968 as amended.https://www.nist.gov/srd/public-law2,3,4,6,7,8-Hexachlorodibenzofuranhttp://www.nist.gov/srd/nist1a.cfm
- SpectraBase2,3,4,6,7,8-HXCDFhttps://spectrabase.com/spectrum/LXGBdX3YxyK2,3,4,6,7,8-Hexachlorodibenzofuranhttps://spectrabase.com/spectrum/HyLQIaF7yiz
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/2,3,4,6,7,8-HexachlorodibenzofuranNORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- PharosLICENSEData accessed from Pharos and TCRD is publicly available from the primary sources listed above. Please respect their individual licenses regarding proper use and redistribution.https://pharos.nih.gov/about2,3,4,6,7,8-Hexachloro-dibenzofuranhttps://pharos.nih.gov/ligands/B3TMUKGWLT39
- Springer Nature
- SpringerMaterials2,3,4,6,7,8-Hexa-chlorodibenzofuranhttps://materials.springer.com/substanceprofile/docs/smsid_rdskhkojvvdrzgzv
- Wikidata2,3,4,6,7,8-hexachlorodibenzofuranhttps://www.wikidata.org/wiki/Q27155434
- PubChem
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- CAMEO ChemicalsLICENSECAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data.https://cameochemicals.noaa.gov/help/reference/terms_and_conditions.htm?d_f=falseCAMEO Chemical Reactivity Classificationhttps://cameochemicals.noaa.gov/browse/react
- EPA Substance Registry ServicesEPA SRS List Classificationhttps://sor.epa.gov/sor_internet/registry/substreg/LandingPage.do
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 403602622https://pubchem.ncbi.nlm.nih.gov/substance/403602622