(2S)-4-(acetylamino)-2-aminobutanoic acid
PubChem CID
441021
Chemical Safety
Molecular Formula
Synonyms
- 1190-46-1
- N-gamma-Acetyldiaminobutyrate
- (2S)-4-(acetylamino)-2-aminobutanoic acid
- (2S)-4-acetamido-2-aminobutanoic acid
- Butanoic acid, 4-(acetylamino)-2-amino-, (2S)-
Molecular Weight
160.17 g/mol
Computed by PubChem 2.2 (PubChem release 2024.11.20)
Dates
- Create:2005-06-24
- Modify:2025-01-18
Description
N(4)-acetyl-L-2,4-diaminobutyric acid is the N(4)-acetyl derivative of L-2,4-diaminobutyric acid It is functionally related to a L-2,4-diaminobutyric acid. It is a tautomer of a N(4)-acetyl-L-2,4-diaminobutyric acid zwitterion.
(2S)-4-acetamido-2-ammoniobutanoate has been reported in Acaciella angustissima with data available.
Chemical Structure Depiction
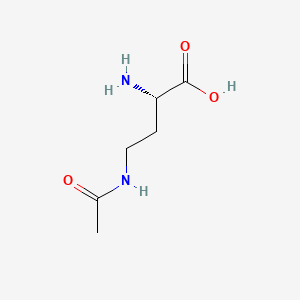
SVG Image

IUPAC Condensed
H-Dab(Ac)(Ac)-OH
Sequence
X
HELM
PEPTIDE1{[CC(=O)NCC[C@@H](C(=O)O)N]}$$$$
(2S)-4-acetamido-2-aminobutanoic acid
Computed by Lexichem TK 2.7.0 (PubChem release 2024.11.20)
InChI=1S/C6H12N2O3/c1-4(9)8-3-2-5(7)6(10)11/h5H,2-3,7H2,1H3,(H,8,9)(H,10,11)/t5-/m0/s1
Computed by InChI 1.07.0 (PubChem release 2024.11.20)
YLZRFVZUZIJABA-YFKPBYRVSA-N
Computed by InChI 1.07.0 (PubChem release 2024.11.20)
CC(=O)NCC[C@@H](C(=O)O)N
Computed by OEChem 2.3.0 (PubChem release 2024.12.12)
C6H12N2O3
Computed by PubChem 2.2 (PubChem release 2024.11.20)
112-590-8
- 1190-46-1
- N-gamma-Acetyldiaminobutyrate
- (2S)-4-(acetylamino)-2-aminobutanoic acid
- (2S)-4-acetamido-2-aminobutanoic acid
- Butanoic acid, 4-(acetylamino)-2-amino-, (2S)-
- N(4)-acetyl-L-2,4-diaminobutyric acid
- 4-N-Acetyl-2,4-diaminobutyric acid
- (S)-4-Acetamido-2-aminobutanoic acid
- N-Acetyl-L-2,4-diaminobutyrate
- N-acetyl-L-2,4-diaminobutanoate
- (2~{S})-4-acetamido-2-azanyl-butanoic acid
- N4-Acetyl-L-2,4-diaminobutanoate
- (2S)-4-acetamido-2-aminobutanoate
- Ngamma-acetyl-L-2,4-diaminobutyric acid
- N4-Acetyl-L-2,4-diaminobutyrate
- 9YT
- MFCD21363201
- N-g-Acetyldiaminobutyric acid
- N(gamma)-Acetyldiaminobutyrate
- CHEBI:7351
- SCHEMBL1768835
- DTXSID30331556
- Ngamma-Acetyl-L-diaminobutyric acid
- (S)-4-Acetamido-2-aminobutanoicacid
- AKOS030212919
- (2S)-2-amino-4-acetamidobutanoic acid
- C06442
- G65197
- Q27107481
Property Name
Property Value
Reference
Property Name
Molecular Weight
Property Value
160.17 g/mol
Reference
Computed by PubChem 2.2 (PubChem release 2024.11.20)
Property Name
XLogP3
Property Value
-4.4
Reference
Computed by XLogP3 3.0 (PubChem release 2024.11.20)
Property Name
Hydrogen Bond Donor Count
Property Value
3
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2024.11.20)
Property Name
Hydrogen Bond Acceptor Count
Property Value
4
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2024.11.20)
Property Name
Rotatable Bond Count
Property Value
4
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2024.11.20)
Property Name
Exact Mass
Property Value
160.08479225 Da
Reference
Computed by PubChem 2.2 (PubChem release 2024.11.20)
Property Name
Monoisotopic Mass
Property Value
160.08479225 Da
Reference
Computed by PubChem 2.2 (PubChem release 2024.11.20)
Property Name
Topological Polar Surface Area
Property Value
92.4 Ų
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2024.11.20)
Property Name
Heavy Atom Count
Property Value
11
Reference
Computed by PubChem
Property Name
Formal Charge
Property Value
0
Reference
Computed by PubChem
Property Name
Complexity
Property Value
158
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2024.11.20)
Property Name
Isotope Atom Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Atom Stereocenter Count
Property Value
1
Reference
Computed by PubChem
Property Name
Undefined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Covalently-Bonded Unit Count
Property Value
1
Reference
Computed by PubChem
Property Name
Compound Is Canonicalized
Property Value
Yes
Reference
Computed by PubChem (release 2021.10.14)
Solid
220 - 222 °C
Follow these links to do a live 2D search or do a live 3D search for this compound, sorted by annotation score. This section is deprecated (see here for details), but these live search links provide equivalent functionality to the table that was previously shown here.
Same Connectivity Count
Same Parent, Connectivity Count
Same Parent, Exact Count
Mixtures, Components, and Neutralized Forms Count
Similar Compounds (2D)
Similar Conformers (3D)
Protein Structures Count
- Cytoplasm
- Extracellular
Pictogram(s)

Signal
Warning
GHS Hazard Statements
H319 (100%): Causes serious eye irritation [Warning Serious eye damage/eye irritation]
Precautionary Statement Codes
P264+P265, P280, P305+P351+P338, and P337+P317
(The corresponding statement to each P-code can be found at the GHS Classification page.)
ECHA C&L Notifications Summary
The GHS information provided by 1 company from 1 notification to the ECHA C&L Inventory.
Eye Irrit. 2 (100%)
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=YLZRFVZUZIJABA-YFKPBYRVSA-N
The LOTUS Initiative for Open Natural Products Research: frozen dataset union wikidata (with metadata) | DOI:10.5281/zenodo.5794106
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/(2S)-4-(Acetylamino)-2-aminobutanoic acidhttps://commonchemistry.cas.org/detail?cas_rn=1190-46-1
- EPA DSSToxN-acetyl-L-2,4-diaminobutanoatehttps://comptox.epa.gov/dashboard/DTXSID30331556CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-notice(2S)-2-Amino-4-acetamidobutanoic acidhttps://echa.europa.eu(2S)-2-Amino-4-acetamidobutanoic acid (EC: 112-590-8)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/398531
- Human Metabolome Database (HMDB)LICENSEHMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications.http://www.hmdb.ca/citing4-Acetamido-2-aminobutanoic acidhttp://www.hmdb.ca/metabolites/HMDB0031411
- ChEBIN(4)-acetyl-L-2,4-diaminobutyric acidhttps://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:7351
- LOTUS - the natural products occurrence databaseLICENSEThe code for LOTUS is released under the GNU General Public License v3.0.https://lotus.nprod.net/(2S)-4-acetamido-2-ammoniobutanoatehttps://www.wikidata.org/wiki/Q27107481LOTUS Treehttps://lotus.naturalproducts.net/
- Japan Chemical Substance Dictionary (Nikkaji)
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.html
- Metabolomics WorkbenchN(4)-acetyl-L-2,4-diaminobutyric acidhttps://www.metabolomicsworkbench.org/data/StructureData.php?RegNo=53313
- Protein Data Bank in Europe (PDBe)
- RCSB Protein Data Bank (RCSB PDB)LICENSEData files contained in the PDB archive (ftp://ftp.wwpdb.org) are free of all copyright restrictions and made fully and freely available for both non-commercial and commercial use. Users of the data should attribute the original authors of that structural data.https://www.rcsb.org/pages/policies
- WikidataN(4)-acetyl-L-2,4-diaminobutyric acid zwitterionhttps://www.wikidata.org/wiki/Q106345569
- PubChem
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 392620210https://pubchem.ncbi.nlm.nih.gov/substance/392620210
- NCBI
CONTENTS