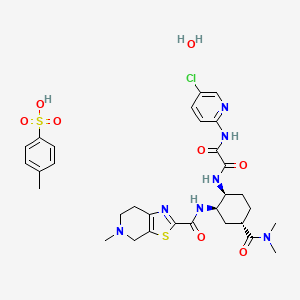
Edoxaban tosylate monohydrate
- 1229194-11-9
- Edoxaban tosylate monohydrate
- edoxaban tosylate hydrate
- Lixiana
- SAVAYSA
- Create:2008-11-10
- Modify:2025-01-18
- DU-176
- DU-176b
- edoxaban
- edoxaban tosylate
- N-(5-chloropyridin-2-yl)-N'-((1S,2R,4S)-4-(N,N-dimethylcarbamoyl)-2-(5-methyl-4,5,6,7- tetrahydro(1,3)thiazolo(5,4-c)pyridine-2-carboxamido)cyclohexyl)oxamide
- N-(5-chloropyridin-2-yl)-N'-((1S,2R,4S)-4-(N,N-dimethylcarbamoyl)-2-(5-methyl-4,5,6,7-tetrahydrothiazolo(5,4-c)pyridine-2-carboxamido)cyclohexyl)ethanediamide p-toluenesulfonate monohydrate
- Savaysa
- 1229194-11-9
- Edoxaban tosylate monohydrate
- edoxaban tosylate hydrate
- Lixiana
- SAVAYSA
- Edoxaban tosilate monohydrate
- Edoxaban tosilate hydrate
- Edoxaban (tosylate monohydrate)
- DU-176b
- UNII-972203R4EW
- Savaysa (TN)
- 972203R4EW
- LIXIANA (TN)
- Ethanediamide, N1-(5-chloro-2-pyridinyl)-N2-[(1S,2R,4S)-4-[(dimethylamino)carbonyl]-2-[[(4,5,6,7-tetrahydro-5-methylthiazolo[5,4-c]pyridin-2-yl)carbonyl]amino]cyclohexyl]-, 4-methylbenzenesulfonate, hydrate (1:1:1)
- 1229194-11-9 (tosylate hydrate)
- N1-(5-Chloropyridin-2-yl)-N2-((1S,2R,4S)-4-(dimethylcarbamoyl)-2-(5-methyl-4,5,6,7-tetrahydrothiazolo[5,4-c]pyridine-2-carboxamido)cyclohexyl)oxalamide 4-methylbenzenesulfonate hydrate
- CHEBI:85974
- Edoxaban tosilate hydrate (JAN)
- DTXSID60153788
- EDOXABAN TOSILATE HYDRATE [JAN]
- EDOXABAN TOSILATE MONOHYDRATE [WHO-DD]
- MFCD28400751
- 4-methylbenzene-1-sulfonic acid--N(1)-(5-chloropyridin-2-yl)-N(2)-{(1S,2R,4S)-4-(dimethylcarbamoyl)-2-[(5-methyl-4,5,6,7-tetrahydro[1,3]thiazolo[5,4-c]pyridine-2-carbonyl)amino]cyclohexyl}ethanediamide--water (1/1/1)
- ETHANEDIAMIDE, N1-(5-CHLORO-2-PYRIDINYL)-N2-((1S,2R,4S)-4-((DIMETHYLAMINO)CARBONYL)-2-(((4,5,6,7-TETRAHYDRO-5-METHYLTHIAZOLO(5,4-C)PYRIDIN-2-YL)CARBONYL)AMINO)CYCLOHEXYL)-, 4-METHYLBENZENESULFONATE, HYDRATE (1:1:1)
- N'-(5-chloropyridin-2-yl)-N-[(1S,2R,4S)-4-(dimethylcarbamoyl)-2-[(5-methyl-6,7-dihydro-4H-[1,3]thiazolo[5,4-c]pyridine-2-carbonyl)amino]cyclohexyl]oxamide;4-methylbenzenesulfonic acid;hydrate
- N-(5-CHLOROPYRIDIN-2-YL)-N'-((1S,2R,4S)-4-((DIMETHYL AMINO)CARBONYL)-2-(((5-METHYL-4,5,6,7-TETRAHYDROTHIAZOLO(5,4-C)PYRIDIN-2-YL)CARBONYL)AMINO)CYCLOHEXYL)ETHANEDIAMIDE P-TOLUENESULFONATE MONOHYDRATE
- N-(5-CHLOROPYRIDIN-2-YL)-N'-((1S,2R,4S)-4-((DIMETHYLAMINO)CARBONYL)-2-(((5-METHYL-4,5,6,7-TETRAHYDROTHIAZOLO(5,4-C)PYRIDIN-2-YL)CARBONYL)AMINO)CYCLOHEXYL)ETHANEDIAMIDE MONO(P-TOLUENESULFONATE) MONOHYDRATE
- 4-methylbenzene-1-sulfonic acid--N(1)-(5-chloropyridin-2-yl)-N(2)-((1S,2R,4S)-4-(dimethylcarbamoyl)-2-((5-methyl-4,5,6,7-tetrahydro(1,3)thiazolo(5,4-c)pyridine-2-carbonyl)amino)cyclohexyl)ethanediamide--water (1/1/1)
- N-(5-Chloropyridin-2-yl)-N'-((1S,2R,4S)-4-(dimethylcarbamoyl)-2-(5-methyl-4,5,6,7-tetrahydro(1,3)thiazolo(5,4-c)pyridine-2-carboxamido)cyclohexyl)oxamide mono(4-methylbenzenesulfonate) monohydrate
- N-(5-Chloropyridin-2-yl)-N'-[(1S,2R,4S)-4-(dimethylcarbamoyl)-2-(5-methyl-4,5,6,7-tetrahydro[1,3]thiazolo[5,4-c]pyridine-2-carboxamido)cyclohexyl]oxamide mono(4-methylbenzenesulfonate) monohydrate
- Edoxaban tosylate monohydrate?
- SCHEMBL330039
- DTXCID8076279
- 480449-71-6, anhydride
- PSMMNJNZVZZNOI-SJILXJHISA-N
- BCP15112
- HY-10264B
- s7280
- AKOS025289705
- edoxaban para-toluene sulfonate hydrate
- CCG-270422
- CCG-270423
- CS-1333
- AC-29306
- AS-35186
- DA-52813
- D09546
- N-(5-Chloropyridin-2-yl)-N'-[(1S,2R,4S)-4-(dimethylcarbamoyl)-2-{[(5-methyl-4,5,6,7-tetrahydro[1,3]thiazolo[5,4-c]pyridin-2-yl)carbonyl]amino}cyclohexyl]ethanediamide p-toluenesulfonate monohydrate
- N1-(5-Chloropyridin-2-yl)-N 2-((1S,2R,4S)-4-[(dimethylamino)carbonyl]-2-{[(5-methyl-4,5,6,7-tetrahydrothiazolo[5,4-c]pyridin-2-yl)carbonyl]amino}cyclohexyl)ethanediamide p-toluenesulfonate monohydrate
- N1-(5-chloropyridin-2-yl)-N2-((1S,2R,4S)-4-[(dimethylamino)carbonyl]-2-{[(5-methyl-4,5, 6,7-tetrahydrothiazolo[5,4-c]pyridin-2-yl)carbonyl]amino}cyclohexyl) ethanediamide p-toluenesulfonate monohydrate
- N1-(5-Chloropyridin-2-yl)-N2-((1S,2R,4S)-4-[(dimethylamino)carbonyl]-2-{[(5-methyl-4,5,6,7-tetrahydrothiazolo [5,4-c]pyridin-2-yl)carbonyl]amino}cyclohexyl)ethanediamide p-toluenesulfonate Monohydrate
- N1-(5-Chloropyridin-2-yl)-N2-((1S,2R,4S)-4-[(dimethylamino)carbonyl]-2-{[(5-methyl-4,5,6,7-tetrahydrothiazolo[5,4-c]pyridin-2-yl)carbonyl]amino}cyclohexyl)ethanediamide mono-p-toluenesulfonate monohydrate
- N1-(5-Chloropyridin-2-yl)-N2-((1S,2R,4S)-4-[(dimethylamino)carbonyl]-2-{[(5-methyl-4,5,6,7-tetrahydrothiazolo[5,4-c]pyridin-2-yl)carbonyl]amino}cyclohexyl)ethanediamide p-toluenesulfonate monohydrate
- N1-(5-chloropyridin-2-yl)-N2-((1S,2R,4S)-4-[(dimethylamino)carbonyl]-2-{[(5-methyl-4,5,6,7-tetrahydrothiazolo[5,4-c]pyridin-2-yl)carbonyl]amino}cyclohexyl)ethanediamide p-toluenesulfonic acid monohydrate
- N1-(5-chloropyridin-2-yl)-N2-[(1S,2R,4S)-4-(dimethylcarbamoyl)-2-{[(5-methyl-4,5,6,7-tetrahydro[1,3]thiazolo[5,4-c]pyridin-2-yl)carbonyl]amino}cyclohexyl]ethanediamide p-toluenesulfonate monohydrate

P260, P319, and P501
(The corresponding statement to each P-code can be found at the GHS Classification page.)
◉ Summary of Use during Lactation
Because no information is available on the use of edoxaban during breastfeeding and the drug is orally absorbable, an alternate drug is preferred, especially while nursing a newborn or preterm infant.
◉ Effects in Breastfed Infants
Relevant published information was not found as of the revision date.
◉ Effects on Lactation and Breastmilk
Relevant published information was not found as of the revision date.
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=PSMMNJNZVZZNOI-SJILXJHISA-N
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/Ethanediamide, N1-(5-chloro-2-pyridinyl)-N2-[(1S,2R,4S)-4-[(dimethylamino)carbonyl]-2-[[(4,5,6,7-tetrahydro-5-methylthiazolo[5,4-c]pyridin-2-yl)carbonyl]amino]cyclohexyl]-, 4-methylbenzenesulfonate, hydrate (1:1:1)https://commonchemistry.cas.org/detail?cas_rn=1229194-11-9
- ChemIDplusEdoxaban tosylate monohydratehttps://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=1229194119ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- EPA DSSToxEdoxaban tosylate monohydratehttps://comptox.epa.gov/dashboard/DTXSID60153788CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-noticeEthanediamide,N1-(5-chloro-2-pyridinyl)-N2-[(1S,2R,4S)-4-[(dimethylamino)carbonyl]-2-[[(4,5,6,7-tetrahydro-5-methylthiazolo[5,4-c]pyridin-2-yl)carbonyl]amino]cyclohexyl]-, 4-methylbenzenesulfonate, hydrate (1:1:1)https://echa.europa.euEthanediamide,N1-(5-chloro-2-pyridinyl)-N2-[(1S,2R,4S)-4-[(dimethylamino)carbonyl]-2-[[(4,5,6,7-tetrahydro-5-methylthiazolo[5,4-c]pyridin-2-yl)carbonyl]amino]cyclohexyl]-, 4-methylbenzenesulfonate, hydrate (1:1:1) (EC: 991-362-7)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/378973
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingEDOXABAN TOSYLATE MONOHYDRATEhttps://gsrs.ncats.nih.gov/ginas/app/beta/substances/972203R4EW
- ChEBIEdoxaban tosylate hydratehttps://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:85974
- ClinicalTrials.govLICENSEThe ClinicalTrials.gov data carry an international copyright outside the United States and its Territories or Possessions. Some ClinicalTrials.gov data may be subject to the copyright of third parties; you should consult these entities for any additional terms of use.https://clinicaltrials.gov/ct2/about-site/terms-conditions#Use
- Drugs and Lactation Database (LactMed)
- Drugs@FDALICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- European Medicines Agency (EMA)LICENSEInformation on the European Medicines Agency's (EMA) website is subject to a disclaimer and copyright and limited reproduction notices.https://www.ema.europa.eu/en/about-us/legal-noticeLixiana (EMEA/H/C/002629)https://www.ema.europa.eu/en/medicines/human/EPAR/lixiana
- EU Clinical Trials Register
- FDA Medication GuidesLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingSAVAYSAhttps://dps.fda.gov/medguide
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.htmlTherapeutic category of drugs in Japanhttp://www.genome.jp/kegg-bin/get_htext?br08301.kegUSP drug classificationhttp://www.genome.jp/kegg-bin/get_htext?br08302.kegAnatomical Therapeutic Chemical (ATC) classificationhttp://www.genome.jp/kegg-bin/get_htext?br08303.kegTarget-based classification of drugshttp://www.genome.jp/kegg-bin/get_htext?br08310.keg
- NIPH Clinical Trials Search of Japan
- Springer Nature
- Therapeutic Target Database (TTD)
- WikidataEdoxaban tosylate monohydratehttps://www.wikidata.org/wiki/Q83020807
- PubChem
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlFactor Xa Inhibitorshttps://www.ncbi.nlm.nih.gov/mesh/68065427
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 403195955https://pubchem.ncbi.nlm.nih.gov/substance/403195955

 CID 10280735 (Edoxaban)
CID 10280735 (Edoxaban) CID 6101 (p-Toluenesulfonic acid)
CID 6101 (p-Toluenesulfonic acid) CID 962 (Water)
CID 962 (Water)