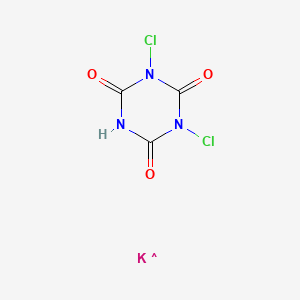
CID 24197163
- Troclosene potassium
- 2244-21-5
- SCHEMBL305053
- AKOS040746424
- Create:2008-02-29
- Modify:2025-01-04
Textiles (Fiber & Fabric Manufacturing) [Category: Industry]
Using Disinfectants or Biocides [Category: Clean]




H272: May intensify fire; oxidizer [Danger Oxidizing liquids; Oxidizing solids]
H302: Harmful if swallowed [Warning Acute toxicity, oral]
H315: Causes skin irritation [Warning Skin corrosion/irritation]
H318: Causes serious eye damage [Danger Serious eye damage/eye irritation]
H332: Harmful if inhaled [Warning Acute toxicity, inhalation]
H335: May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation]
H410: Very toxic to aquatic life with long lasting effects [Warning Hazardous to the aquatic environment, long-term hazard]
P210, P220, P261, P264, P264+P265, P270, P271, P273, P280, P301+P317, P302+P352, P304+P340, P305+P354+P338, P317, P319, P321, P330, P332+P317, P362+P364, P370+P378, P391, P403+P233, P405, and P501
(The corresponding statement to each P-code can be found at the GHS Classification page.)
Acute toxicity - category 4
Acute toxicity - category 4
Skin irritation - category 2
Eye damage - category 1
Specific target organ toxicity (single exposure) - category 3
Oxidising solid - category 2
Hazardous to the aquatic environment (chronic) - category 1
Hazardous to the aquatic environment (acute) - category 1

Special Hazards of Combustion Products: May form toxic chlorine and other gases in fire
Behavior in Fire: Decomposition can be initiated with a heat source and can propagate throughout the mass with the evolution of dense fumes. Containers may explode when heated. (USCG, 1999)
EYES: First check the victim for contact lenses and remove if present. Flush victim's eyes with water or normal saline solution for 20 to 30 minutes while simultaneously calling a hospital or poison control center. Do not put any ointments, oils, or medication in the victim's eyes without specific instructions from a physician. IMMEDIATELY transport the victim after flushing eyes to a hospital even if no symptoms (such as redness or irritation) develop.
SKIN: IMMEDIATELY flood affected skin with water while removing and isolating all contaminated clothing. Gently wash all affected skin areas thoroughly with soap and water. If symptoms such as redness or irritation develop, IMMEDIATELY call a physician and be prepared to transport the victim to a hospital for treatment.
INHALATION: IMMEDIATELY leave the contaminated area; take deep breaths of fresh air. If symptoms (such as wheezing, coughing, shortness of breath, or burning in the mouth, throat, or chest) develop, call a physician and be prepared to transport the victim to a hospital. Provide proper respiratory protection to rescuers entering an unknown atmosphere. Whenever possible, Self-Contained Breathing Apparatus (SCBA) should be used; if not available, use a level of protection greater than or equal to that advised under Protective Clothing.
INGESTION: DO NOT INDUCE VOMITING. If the victim is conscious and not convulsing, give 1 or 2 glasses of water to dilute the chemical and IMMEDIATELY call a hospital or poison control center. Be prepared to transport the victim to a hospital if advised by a physician. If the victim is convulsing or unconscious, do not give anything by mouth, ensure that the victim's airway is open and lay the victim on his/her side with the head lower than the body. DO NOT INDUCE VOMITING. IMMEDIATELY transport the victim to a hospital. (NTP, 1992)
Excerpt from ERG Guide 140 [Oxidizers]:
IMMEDIATE PRECAUTIONARY MEASURE: Isolate spill or leak area in all directions for at least 50 meters (150 feet) for liquids and at least 25 meters (75 feet) for solids.
LARGE SPILL: Consider initial downwind evacuation for at least 100 meters (330 feet).
FIRE: If tank, rail tank car or highway tank is involved in a fire, ISOLATE for 800 meters (1/2 mile) in all directions; also, consider initial evacuation for 800 meters (1/2 mile) in all directions. If ammonium nitrate products are in a tank, rail car or truck and involved in a fire, ISOLATE for 1600 meters (1 mile) in all directions; also, initiate evacuation including emergency responders for 1600 meters (1 mile) in all directions. (ERG, 2024)
Excerpt from ERG Guide 140 [Oxidizers]:
Keep combustibles (wood, paper, oil, etc.) away from spilled material. Do not touch damaged containers or spilled material unless wearing appropriate protective clothing. Stop leak if you can do it without risk. Do not get water inside containers.
SMALL DRY SPILL: With clean shovel, place material into clean, dry container and cover loosely; move containers from spill area.
SMALL LIQUID SPILL: Use a non-combustible material like vermiculite or sand to soak up the product and place into a container for later disposal.
LARGE SPILL: Dike far ahead of liquid spill for later disposal. (ERG, 2024)
Salts, Acidic
Acyl Halides, Sulfonyl Halides, and Chloroformates
Oxidizing Agents, Strong
Halogenating Agents
Strong Oxidizing Agent
Water-Reactive
Dermatotoxin - Skin burns.
Lacrimator (Lachrymator) - A substance that irritates the eyes and induces the flow of tears.
Toxic Pneumonitis - Inflammation of the lungs induced by inhalation of metal fumes or toxic gases and vapors.
- Australian Industrial Chemicals Introduction Scheme (AICIS)1,3,5-Triazine-2,4,6(1H,3H,5H)-trione, 1,3-dichloro-, potassium salthttps://services.industrialchemicals.gov.au/search-assessments/1,3,5-Triazine-2,4,6(1H,3H,5H)-trione, 1,3-dichloro-, potassium salthttps://services.industrialchemicals.gov.au/search-inventory/
- CAMEO ChemicalsLICENSECAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data.https://cameochemicals.noaa.gov/help/reference/terms_and_conditions.htm?d_f=falsePOTASSIUM DICHLORO-S-TRIAZINETRIONE, DRYhttps://cameochemicals.noaa.gov/chemical/9011CAMEO Chemical Reactivity Classificationhttps://cameochemicals.noaa.gov/browse/react
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/Potassium dichloroisocyanuratehttps://commonchemistry.cas.org/detail?cas_rn=2244-21-5
- EPA Chemicals under the TSCA1,3,5-Triazine-2,4,6(1H,3H,5H)-trione, 1,3-dichloro-, potassium salt (1:1)https://www.epa.gov/chemicals-under-tscaEPA TSCA Classificationhttps://www.epa.gov/tsca-inventory
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-noticeTroclosene potassiumhttps://echa.europa.eu/substance-information/-/substanceinfo/100.017.118
- Hazardous Substances Data Bank (HSDB)POTASSIUM DICHLOROISOCYANURATEhttps://pubchem.ncbi.nlm.nih.gov/source/hsdb/5871
- New Zealand Environmental Protection Authority (EPA)LICENSEThis work is licensed under the Creative Commons Attribution-ShareAlike 4.0 International licence.https://www.epa.govt.nz/about-this-site/general-copyright-statement/1,3,5-Triazine-2,4,6(1H,3H,5H)-trione, 1,3-dichloro-, potassium salthttps://www.epa.govt.nz/industry-areas/hazardous-substances/guidance-for-importers-and-manufacturers/hazardous-substances-databases/
- NJDOH RTK Hazardous Substance Listpotassium dichloroisocyanuratehttp://nj.gov/health/eoh/rtkweb/documents/fs/1563.pdf
- Haz-Map, Information on Hazardous Chemicals and Occupational DiseasesLICENSECopyright (c) 2022 Haz-Map(R). All rights reserved. Unless otherwise indicated, all materials from Haz-Map are copyrighted by Haz-Map(R). No part of these materials, either text or image may be used for any purpose other than for personal use. Therefore, reproduction, modification, storage in a retrieval system or retransmission, in any form or by any means, electronic, mechanical or otherwise, for reasons other than personal use, is strictly prohibited without prior written permission.https://haz-map.com/AboutPotassium dichloroisocyanuratehttps://haz-map.com/Agents/6793
- EPA Pesticide Ecotoxicity Database
- Hazardous Chemical Information System (HCIS), Safe Work Australiatroclosene potassiumhttp://hcis.safeworkaustralia.gov.au/HazardousChemical/Details?chemicalID=4690
- Regulation (EC) No 1272/2008 of the European Parliament and of the CouncilLICENSEThe copyright for the editorial content of this source, the summaries of EU legislation and the consolidated texts, which is owned by the EU, is licensed under the Creative Commons Attribution 4.0 International licence.https://eur-lex.europa.eu/content/legal-notice/legal-notice.htmltroclosene potassiumhttps://eur-lex.europa.eu/eli/reg/2008/1272/oj
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.html
- NCI Thesaurus (NCIt)LICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuse
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/NORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- WikidataCID 24197163https://www.wikidata.org/wiki/Q126605693
- PubChem
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
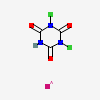

 CID 16726 (Troclosene)
CID 16726 (Troclosene) CID 5462222 (Potassium)
CID 5462222 (Potassium)