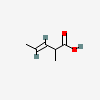2-Methyl-3-pentenoic acid, (3E)-
- 2-Methyl-3-pentenoic acid
- 2-Methyl-trans-3-pentenoic acid
- FEMA No. 3464, E-
- UNII-2ZQ5618KOJ
- 2-Methyl-3-pentenoic acid, (3E)-
- Create:2006-04-28
- Modify:2025-01-11
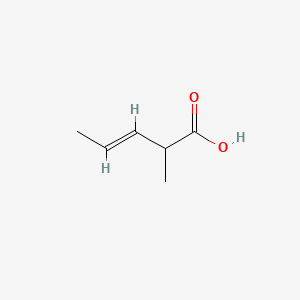
- 2-Methyl-3-pentenoic acid
- 2-Methyl-trans-3-pentenoic acid
- FEMA No. 3464, E-
- UNII-2ZQ5618KOJ
- 2-Methyl-3-pentenoic acid, (3E)-
- 2ZQ5618KOJ
- 3-Pentenoic acid, 2-methyl-, (E)-
- 3-Pentenoic acid, 2-methyl-, (3E)-
- (+/-)-2-methyl-trans-3-pentenoic acid
- 2-Methyl-trans-3-pentenoic acid, (+/-)-
- 2-Methylpent-3-en-1-oic acid
- 55894-37-6
- FEMA No. 3464
- UNII-7N2Z33Q5NJ
- (E)-2-methylpent-3-enoic acid
- EINECS 253-610-6
- methyl-3-pentenoic acid
- SCHEMBL317016
- 7N2Z33Q5NJ
- CHEBI:173389
- NFRJJFMXYKSRPK-ONEGZZNKSA-N
- (3E)-2-methyl-3-pentenoic acid
- AC9353
- AKOS006293940
- NS00012962
- Q27255863

P260, P264, P280, P301+P330+P331, P302+P361+P354, P304+P340, P305+P354+P338, P316, P321, P363, P405, and P501
(The corresponding statement to each P-code can be found at the GHS Classification page.)
Aggregated GHS information provided per 1375 reports by companies from 3 notifications to the ECHA C&L Inventory.
Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown.
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=NFRJJFMXYKSRPK-ONEGZZNKSA-N
- ChEBI2-Methyl-3-pentenoic acidhttps://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:173389
- ChemIDplus2-Methyl-3-pentenoic acidhttps://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=00376746382-Methyl-3-pentenoic acid, (3E)-https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0055894376ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-notice2-methylpent-3-en-1-oic acidhttps://echa.europa.eu/substance-information/-/substanceinfo/100.048.7192-methylpent-3-en-1-oic acid (EC: 253-610-6)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/56182
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking2-METHYL-3-PENTENOIC ACID, (3E)-https://gsrs.ncats.nih.gov/ginas/app/beta/substances/2ZQ5618KOJ
- FDA Substances Added to FoodLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- Flavor and Extract Manufacturers Association (FEMA)2-METHYL-3-PENTENOIC ACIDhttps://www.femaflavor.org/flavor-library/2-methyl-3-pentenoic-acid
- Joint FAO/WHO Expert Committee on Food Additives (JECFA)LICENSEPermission from WHO is not required for the use of WHO materials issued under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Intergovernmental Organization (CC BY-NC-SA 3.0 IGO) licence.https://www.who.int/about/policies/publishing/copyrightMethyl-3-pentenoic acidhttps://www.fao.org/food/food-safety-quality/scientific-advice/jecfa/jecfa-flav/details/en/c/659/2-METHYL-3-PENTENOIC ACIDhttps://apps.who.int/food-additives-contaminants-jecfa-database/Home/Chemical/2465
- Japan Chemical Substance Dictionary (Nikkaji)
- NIST Mass Spectrometry Data CenterLICENSEData covered by the Standard Reference Data Act of 1968 as amended.https://www.nist.gov/srd/public-law2-methyl-3-pentenoic acidhttp://www.nist.gov/srd/nist1a.cfm
- Springer Nature
- Thieme ChemistryLICENSEThe Thieme Chemistry contribution within PubChem is provided under a CC-BY-NC-ND 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc-nd/4.0/
- Wikidata(3E)-2-methyl-3-pentenoic acidhttps://www.wikidata.org/wiki/Q27255863
- PubChem
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/NORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 403385563https://pubchem.ncbi.nlm.nih.gov/substance/403385563