Undecanedioic Acid
- UNDECANEDIOIC ACID
- 1852-04-6
- 1,9-Nonanedicarboxylic acid
- 1,9-Nonanedicarboxylicacid
- 1,11-Undecanedioic acid
- Create:2005-03-26
- Modify:2025-01-04
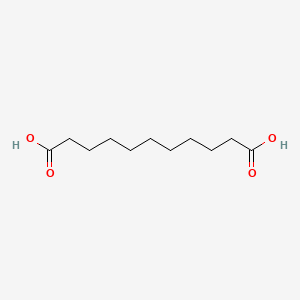
- UNDECANEDIOIC ACID
- 1852-04-6
- 1,9-Nonanedicarboxylic acid
- 1,9-Nonanedicarboxylicacid
- 1,11-Undecanedioic acid
- Hendecanedioic acid
- Undecanedionic acid
- Undecandioic acid
- Hendecanedioate
- Undecanedioate
- Undecanedionate
- UNII-7059GFK8NV
- 7059GFK8NV
- DTXSID0044862
- 1,11-Undecanedioate
- CHEBI:73713
- 1,9-Nonanedicarboxylate
- EINECS 217-440-6
- MFCD00004444
- NSC 400241
- NSC-400241
- DTXCID8024862
- EC 217-440-6
- NSC400241
- 1052-04-6
- Undecandioic acid; 1,11-Undecanedioic acid
- Nonamethylenedicarboxylic Acid
- 1,9-Nonanedicarboxylic Acid; Hendecanedioic Acid; NSC 400241
- undecandisäure
- Undecanedioic acid, 97%
- bmse000847
- bmse000880
- Undecane-1,11-dioic acid
- SCHEMBL28401
- CHEMBL3183300
- Tox21_300954
- LMFA01170007
- s6333
- AKOS015919038
- CS-W014841
- HY-W014125
- NCGC00248227-01
- NCGC00254856-01
- AS-14889
- SY051603
- CAS-1852-04-6
- N0333
- NS00002110
- Undecanedioic acid, puriss., >=99.0% (GC)
- EN300-1212389
- W-109719
- Q27144063
- Z1508914846
57.0346 100
80.0268 75.78
153.1284 58.96
151.1128 33.43
153.1285 100
197.1183 38.94
57.0346 21.62
151.1129 20.42
80.0267 20.02
215.1288 999
197.1184 538
153.1285 228
171.1393 7
125.0974 2
197.1183 999
215.1288 840
153.1285 608
171.1391 17
125.0972 7
- Corrosion inhibitor
- Paint additives and coating additives not described by other categories
- Lubricants and lubricant additives
- Plasticizers
- Corrosion inhibitors and anti-scaling agents
- Adhesives and sealant chemicals
2019: 1,000,000 lb - <20,000,000 lb
2018: 1,000,000 - <10,000,000 lb
2017: <1,000,000 lb
2016: 1,000,000 lb - <20,000,000 lb
- Soap, Cleaning Compound, and Toilet Preparation Manufacturing
- Plastics Material and Resin Manufacturing
- All Other Chemical Product and Preparation Manufacturing
- Adhesive Manufacturing
- Paint and Coating Manufacturing
- Wholesale and Retail Trade
- Petroleum Lubricating Oil and Grease Manufacturing

H315 (14.2%): Causes skin irritation [Warning Skin corrosion/irritation]
H319 (99.1%): Causes serious eye irritation [Warning Serious eye damage/eye irritation]
P264, P264+P265, P280, P302+P352, P305+P351+P338, P321, P332+P317, P337+P317, and P362+P364
(The corresponding statement to each P-code can be found at the GHS Classification page.)
Aggregated GHS information provided per 695 reports by companies from 9 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies.
Reported as not meeting GHS hazard criteria per 6 of 695 reports by companies. For more detailed information, please visit ECHA C&L website.
There are 8 notifications provided by 689 of 695 reports by companies with hazard statement code(s).
Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown.
Skin Irrit. 2 (14.2%)
Eye Irrit. 2A (99.1%)
Skin Irrit. 2 (100%)
Eye Irrit. 2 (100%)
Chemical: Undecanedioic acid
Specific Information Requirement: Obligations to provide information apply. You must tell us within 28 days if the circumstances of your importation or manufacture (introduction) are different to those in our assessment.
Silke Matysik, Caroline Ivanne Le Roy, Gerhard Liebisch, Sandrine Paule Claus. Metabolomics of fecal samples: A practical consideration. Trends in Food Science & Technology. Vol. 57, Part B, Nov. 2016, p.244-255: http://www.sciencedirect.com/science/article/pii/S0924224416301984
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=LWBHHRRTOZQPDM-UHFFFAOYSA-N
- Australian Industrial Chemicals Introduction Scheme (AICIS)Undecanedioic acidhttps://services.industrialchemicals.gov.au/search-inventory/
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/Undecanedioic acidhttps://commonchemistry.cas.org/detail?cas_rn=1852-04-6
- ChemIDplusChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- DTP/NCILICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuse
- EPA Chemical Data Reporting (CDR)LICENSEThe U.S. Government retains a nonexclusive, royalty-free license to publish or reproduce these documents, or allow others to do so, for U.S. Government purposes. These documents may be freely distributed and used for non-commercial, scientific and educational purposes.https://www.epa.gov/web-policies-and-procedures/epa-disclaimers#copyrightUndecanedioic acidhttps://www.epa.gov/chemical-data-reporting
- EPA Chemicals under the TSCAUndecanedioic acidhttps://www.epa.gov/chemicals-under-tscaEPA TSCA Classificationhttps://www.epa.gov/tsca-inventory
- EPA DSSToxUndecanedioic acidhttps://comptox.epa.gov/dashboard/DTXSID0044862CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-noticeUndecanedioic acidhttps://chem.echa.europa.eu/100.015.855undecanedioic acidhttps://echa.europa.eu/substance-information/-/substanceinfo/100.233.557Undecanedioic acid (EC: 217-440-6)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/25327undecanedioic acid (EC: 806-507-0)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/241903
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingUNDECANEDIOIC ACIDhttps://gsrs.ncats.nih.gov/ginas/app/beta/substances/7059GFK8NV
- Human Metabolome Database (HMDB)LICENSEHMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications.http://www.hmdb.ca/citingUndecanedioic acidhttp://www.hmdb.ca/metabolites/HMDB0000888HMDB0000888_nmr_one_1585https://hmdb.ca/metabolites/HMDB0000888#spectra
- New Zealand Environmental Protection Authority (EPA)LICENSEThis work is licensed under the Creative Commons Attribution-ShareAlike 4.0 International licence.https://www.epa.govt.nz/about-this-site/general-copyright-statement/
- ChEBIUndecanedioic acidhttps://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:73713
- LOTUS - the natural products occurrence databaseLICENSEThe code for LOTUS is released under the GNU General Public License v3.0.https://lotus.nprod.net/Undecanedioic acidhttps://www.wikidata.org/wiki/Q27144063LOTUS Treehttps://lotus.naturalproducts.net/
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.html
- Crystallography Open Database (COD)LICENSEAll data in the COD and the database itself are dedicated to the public domain and licensed under the CC0 License. Users of the data should acknowledge the original authors of the structural data.https://creativecommons.org/publicdomain/zero/1.0/
- FooDBLICENSEFooDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (FooDB) and the original publication.https://foodb.ca/aboutUndecanedioic acidhttps://foodb.ca/compounds/FDB022300
- SpectraBaseundecanedioic acidhttps://spectrabase.com/spectrum/GLCzAV1jM6oUndecanedioic acidhttps://spectrabase.com/spectrum/Jo8ahfmtIO1Undecanedioic acidhttps://spectrabase.com/spectrum/21SnC7NpLmTUndecanedioic acidhttps://spectrabase.com/spectrum/2N9EzVqpx80UNDECANEDIOIC ACIDhttps://spectrabase.com/spectrum/8hTJGHm31HEUNDECANEDIOIC ACIDhttps://spectrabase.com/spectrum/J2OO5oEk0kkUndecanedioic acidhttps://spectrabase.com/spectrum/FTKeWSmi1k9UNDECANEDIOIC ACIDhttps://spectrabase.com/spectrum/El7HwlzhLjCUndecanedioic acidhttps://spectrabase.com/spectrum/8PHaI8mz9YJUndecanedioic acidhttps://spectrabase.com/spectrum/FUw0psRrG4i
- NIST Mass Spectrometry Data CenterLICENSEData covered by the Standard Reference Data Act of 1968 as amended.https://www.nist.gov/srd/public-lawUndecanedioic acidhttp://www.nist.gov/srd/nist1a.cfm
- Japan Chemical Substance Dictionary (Nikkaji)
- LIPID MAPSLipid Classificationhttps://www.lipidmaps.org/
- Natural Product Activity and Species Source (NPASS)Undecanedioic Acidhttps://bidd.group/NPASS/compound.php?compoundID=NPC294085
- West Coast Metabolomics Center-UC DavisUndecanedioic acid
- MassBank Europe
- MassBank of North America (MoNA)LICENSEThe content of the MoNA database is licensed under CC BY 4.0.https://mona.fiehnlab.ucdavis.edu/documentation/license
- Metabolomics WorkbenchUndecanedioic acidhttps://www.metabolomicsworkbench.org/data/StructureData.php?RegNo=1931
- Springer Nature
- SpringerMaterials
- Thieme ChemistryLICENSEThe Thieme Chemistry contribution within PubChem is provided under a CC-BY-NC-ND 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc-nd/4.0/
- Wikidataundecanedioic acidhttps://www.wikidata.org/wiki/Q27144063
- PubChem
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/NORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- EPA Substance Registry ServicesEPA SRS List Classificationhttps://sor.epa.gov/sor_internet/registry/substreg/LandingPage.do
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 403385807https://pubchem.ncbi.nlm.nih.gov/substance/403385807
- NCBI