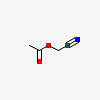
Cyanomethyl acetate
- Cyanomethyl acetate
- 1001-55-4
- Acetic acid cyanomethyl ester
- Acetoxyacetonitrile
- (ACETYLOXY)ACETONITRILE
- Create:2005-03-26
- Modify:2025-01-04
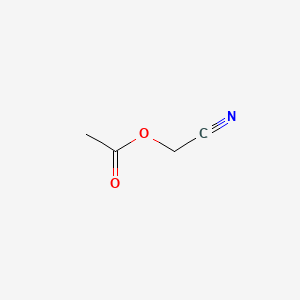
- Cyanomethyl acetate
- 1001-55-4
- Acetic acid cyanomethyl ester
- Acetoxyacetonitrile
- (ACETYLOXY)ACETONITRILE
- cyanomethylacetate
- 2-Acetoxyacetonitrile
- Glycolonitrile, acetate
- Glycolonitrile, acetate (ester)
- Acetonitrile, (acetyloxy)-
- VM1DZ0X2D6
- NSC-71942
- Acetyloxyacetonitrile
- HSDB 2124
- NSC 71942
- UNII-VM1DZ0X2D6
- Kyanmethylester kyseliny octove [Czech]
- BRN 1745113
- Kyanmethylester kyseliny octove
- acetonitrile acetate
- ACETOXYMETHYL CYANIDE
- WLN: NC1OV1
- 3-03-00-00401 (Beilstein Handbook Reference)
- DTXSID00142939
- AJEHNBIPLQJTNU-UHFFFAOYSA-N
- NSC71942
- MFCD00242714
- (ACETYLOXY)ACETONITRILE [HSDB]
- ACETONITRILE, 2-(ACETYLOXY)-
- AKOS027473379
- CS-0248523
- EN300-40098
- G32186
- Q27291897


H227 (100%): Combustible liquid [Warning Flammable liquids]
H300 (100%): Fatal if swallowed [Danger Acute toxicity, oral]
H310 (100%): Fatal in contact with skin [Danger Acute toxicity, dermal]
H315 (100%): Causes skin irritation [Warning Skin corrosion/irritation]
H319 (100%): Causes serious eye irritation [Warning Serious eye damage/eye irritation]
H330 (100%): Fatal if inhaled [Danger Acute toxicity, inhalation]
H335 (100%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation]
P210, P260, P261, P262, P264, P264+P265, P270, P271, P280, P284, P301+P316, P302+P352, P304+P340, P305+P351+P338, P316, P319, P320, P321, P330, P332+P317, P337+P317, P361+P364, P362+P364, P370+P378, P403, P403+P233, P405, and P501
(The corresponding statement to each P-code can be found at the GHS Classification page.)
Flam. Liq. 4 (100%)
Acute Tox. 2 (100%)
Acute Tox. 2 (100%)
Skin Irrit. 2 (100%)
Eye Irrit. 2A (100%)
Acute Tox. 1 (100%)
STOT SE 3 (100%)
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=AJEHNBIPLQJTNU-UHFFFAOYSA-N
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/2-(Acetyloxy)acetonitrilehttps://commonchemistry.cas.org/detail?cas_rn=1001-55-4
- ChemIDplus(Acetyloxy)acetonitrilehttps://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0001001554ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- DTP/NCILICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuse
- EPA DSSTox(Acetyloxy)acetonitrilehttps://comptox.epa.gov/dashboard/DTXSID00142939CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-noticecyanomethyl acetatehttps://echa.europa.eu/substance-information/-/substanceinfo/100.334.930cyanomethyl acetate (EC: 872-138-7)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/319672
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingCYANOMETHYLACETATEhttps://gsrs.ncats.nih.gov/ginas/app/beta/substances/VM1DZ0X2D6
- Hazardous Substances Data Bank (HSDB)(ACETYLOXY)ACETONITRILEhttps://pubchem.ncbi.nlm.nih.gov/source/hsdb/2124
- Haz-Map, Information on Hazardous Chemicals and Occupational DiseasesLICENSECopyright (c) 2022 Haz-Map(R). All rights reserved. Unless otherwise indicated, all materials from Haz-Map are copyrighted by Haz-Map(R). No part of these materials, either text or image may be used for any purpose other than for personal use. Therefore, reproduction, modification, storage in a retrieval system or retransmission, in any form or by any means, electronic, mechanical or otherwise, for reasons other than personal use, is strictly prohibited without prior written permission.https://haz-map.com/About(Acetyloxy)acetonitrilehttps://haz-map.com/Agents/3432
- Japan Chemical Substance Dictionary (Nikkaji)
- SpectraBaseACETIC ACID, CYANOMETHYL ESTERhttps://spectrabase.com/spectrum/BjODisOmrSyAcetic acid, cyanomethyl esterhttps://spectrabase.com/spectrum/3M5NFY6eBgIacetic acid, cyanomethyl esterhttps://spectrabase.com/spectrum/3Iv1F9SqEuDACETIC ACID, CYANOMETHYL ESTERhttps://spectrabase.com/spectrum/DoVJpIvhhdPACETIC ACID, CYANOMETHYL ESTERhttps://spectrabase.com/spectrum/4tN5fM8bAszAcetonitrile, (acetyloxy)-https://spectrabase.com/spectrum/Git0wosaPZ7
- Springer Nature
- Thieme ChemistryLICENSEThe Thieme Chemistry contribution within PubChem is provided under a CC-BY-NC-ND 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc-nd/4.0/
- Wikidatacyanomethylacetatehttps://www.wikidata.org/wiki/Q27291897
- PubChem
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 388852429https://pubchem.ncbi.nlm.nih.gov/substance/388852429