Leucovorin calcium pentahydrate
- Leucovorin calcium pentahydrate
- 6035-45-6
- Folinic acid calcium salt pentahydrate
- Calcium folinate pentahydrate
- Leucovorin calcium hydrate
- Create:2019-01-18
- Modify:2025-01-04
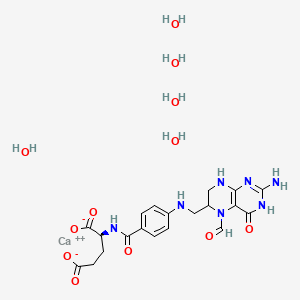
- 5 Formyltetrahydrofolate
- 5 Formyltetrahydropteroylglutamate
- 5-Formyltetrahydrofolate
- 5-Formyltetrahydropteroylglutamate
- Acid, Folinic
- Calcium Folinate
- Calcium Leucovorin
- Citrovorum Factor
- Factor, Citrovorum
- Folinate, Calcium
- Folinic Acid
- Folinic Acid SF
- Folinic Acid-SF
- Leucovorin
- Leucovorin, (D)-Isomer
- Leucovorin, (DL)-Isomer
- Leucovorin, (R)-Isomer
- Leucovorin, Calcium
- Leucovorin, Calcium (1:1) Salt
- Leucovorin, Calcium (1:1) Salt, (DL)-Isomer
- Leucovorin, Calcium (1:1) Salt, Pentahydrate
- Leucovorin, Monosodium Salt
- Leukovorin
- Leukovorum
- Monosodium Salt Leucovorin
- N(5)-Formyltetrahydrofolate
- Wellcovorin
- Leucovorin calcium pentahydrate
- 6035-45-6
- Folinic acid calcium salt pentahydrate
- Calcium folinate pentahydrate
- Leucovorin calcium hydrate
- R3W57OBQ5W
- DTXSID3036999
- Leucovorin calcium salt pentahydrate
- Rescufolin
- Wellcovorin
- Folinic acid (calcium salt pentahydrate)
- FOLINIC ACID, CALCIUM SALT PENTAHYDRATE
- Calcium (2S)-2-(4-(((2-amino-5-formyl-4-oxo-1,4,5,6,7,8-hexahydropteridin-6-yl)methyl)amino)benzamido)pentanedioate pentahydrate
- calcium;(2S)-2-[[4-[(2-amino-5-formyl-4-oxo-1,6,7,8-tetrahydropteridin-6-yl)methylamino]benzoyl]amino]pentanedioate;pentahydrate
- calcium;(2S)-2-[[4-[(2-amino-5-formyl-4-oxo-3,6,7,8-tetrahydropteridin-6-yl)methylamino]benzoyl]amino]pentanedioate;pentahydrate
- UNII-R3W57OBQ5W
- NCGC00017055-01
- CAS-6035-45-6
- CHEMBL3184463
- DTXCID1016999
- CALCIUM FOLINATE [WHO-IP]
- HY-B0080
- LEUCOVORIN CALCIUM [WHO-IP]
- Tox21_110760
- s1236
- CALCII FOLINAS [WHO-IP LATIN]
- CCG-270199
- CS-1777
- L-Glutamic acid, N-(4-(((2-amino-5-formyl-1,4,5,6,7,8-hexahydro-4-oxo-6-pteridinyl)methyl)amino)benzoyl)-, calcium salt (1:1), pentahydrate
- CALCIUM FOLINATE PENTAHYDRATE [WHO-DD]
- AB01568259_01
- FOLINIC ACID CALCIUM SALT PENTAHYDRATE [MI]
- Q27287765
- Calcium (2S)-2-(4-(((2-amino-5-formyl-4-oxo-1,4,5,6,7,8-hexahydropteridin-6-yl)methyl)amino)benzamido)pentanedioate
- CALCIUM N-(P-(((2-AMINO-5-FORMYL-5,6,7,8-TETRAHYDRO-4-HYDROXY-6-PTERIDINYL)METHYL)AMINO)BENZOYL)-L-GLUTAMATE (1:1) PENTAHYDRATE [WHO-IP]
- Glutamic acid, N-(p-(((2-amino-5-formyl-5,6,7,8-tetrahydro-4-hydroxy-6-pteridinyl)methyl)amino)benzoyl)-, calcium salt, pentahydrate, L-
- L-GLUTAMIC ACID, N-(4-(((2-AMINO-5-FORMYL-3,4,5,6,7,8-HEXAHYDRO-4-OXO-6-PTERIDINYL)METHYL)AMINO)BENZOYL)-, CALCIUM SALT, HYDRATE (1:1:5)
Use (kg; approx.) in Germany (2009): >10
Consumption (g per capita; approx.) in Germany (2009): 0.000122
Calculated removal (%): 22


H315 (100%): Causes skin irritation [Warning Skin corrosion/irritation]
H317 (100%): May cause an allergic skin reaction [Warning Sensitization, Skin]
H319 (97.5%): Causes serious eye irritation [Warning Serious eye damage/eye irritation]
H334 (97.5%): May cause allergy or asthma symptoms or breathing difficulties if inhaled [Danger Sensitization, respiratory]
H335 (97.5%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation]
P233, P260, P261, P264, P264+P265, P271, P272, P280, P284, P302+P352, P304+P340, P305+P351+P338, P319, P321, P332+P317, P333+P317, P337+P317, P342+P316, P362+P364, P403, P403+P233, P405, and P501
(The corresponding statement to each P-code can be found at the GHS Classification page.)
Aggregated GHS information provided per 40 reports by companies from 3 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies.
Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown.
Skin Irrit. 2 (100%)
Skin Sens. 1 (100%)
Eye Irrit. 2 (97.5%)
Resp. Sens. 1 (97.5%)
STOT SE 3 (97.5%)
Skin Irrit. 2 (100%)
Skin Sens. 1A (100%)
Eye Irrit. 2 (100%)
Resp. Sens. 1A (100%)
STOT SE 3 (100%)
◉ Summary of Use during Lactation
Leucovorin (folinic acid; 5-formyltetrahydrofolic acid) and its levo- isomer, levoleucovorin, are folic acid derivatives that are normal components of breastmilk. Because leucovorin and levoleucovorin are used as therapeutic agents with potentially toxic drugs such as fluorouracil or methotrexate, the LactMed record of the drug it is used with should be consulted.
◉ Effects in Breastfed Infants
Relevant published information was not found as of the revision date.
◉ Effects on Lactation and Breastmilk
Relevant published information was not found as of the revision date.
- Australian Industrial Chemicals Introduction Scheme (AICIS)L-Glutamic acid, N-[4-[[(2-amino-5-formyl-1,4,5,6,7,8-hexahydro-4-oxo-6-pteridinyl)methyl]amino]benzoyl]-, calcium salt(1:1), pentahydratehttps://services.industrialchemicals.gov.au/search-inventory/
- ChemIDplusLeucovorin calcium pentahydratehttps://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0006035456ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- EPA DSSToxCalcium folinate pentahydratehttps://comptox.epa.gov/dashboard/DTXSID3036999CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-noticeFolinic acid calcium salt hydratehttps://echa.europa.eu/substance-information/-/substanceinfo/100.151.674Folinic acid calcium salt pentahydratehttps://echa.europa.eu/substance-information/-/substanceinfo/100.283.798Folinic acid calcium salt hydrate (EC: 622-950-0)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/156273Folinic acid calcium salt pentahydrate (EC: 846-741-0)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/292521
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingLEUCOVORIN CALCIUM PENTAHYDRATEhttps://gsrs.ncats.nih.gov/ginas/app/beta/substances/R3W57OBQ5W
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.html
- Drugs and Lactation Database (LactMed)
- Drugs@FDALICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- EU Clinical Trials Register
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.html
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/SODIUM FOLINATE
- Wikidataleucovorin calcium pentahydratehttps://www.wikidata.org/wiki/Q27287765
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlVitamin B Complexhttps://www.ncbi.nlm.nih.gov/mesh/68014803
- PubChem
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html

 CID 135403648 (Leucovorin)
CID 135403648 (Leucovorin) CID 962 (Water)
CID 962 (Water) CID 5460341 (Calcium)
CID 5460341 (Calcium)