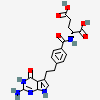
Pemetrexed, (R)-
- (R)-Pemetrexed
- 182009-04-7
- Pemetrexed, (R)-
- Pemetrexed (R) enantiomer
- D-Pemetrexed Hydrate
- Create:2019-01-15
- Modify:2025-01-04
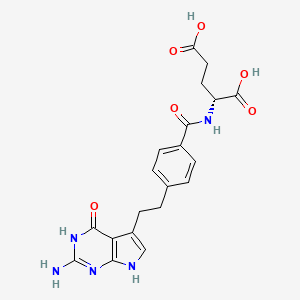
- (R)-Pemetrexed
- 182009-04-7
- Pemetrexed, (R)-
- Pemetrexed (R) enantiomer
- D-Pemetrexed Hydrate
- FG8Q47888S
- (2R)-2-{[4-(2-{2-amino-4-oxo-3H,4H,7H-pyrrolo[2,3-d]pyrimidin-5-yl}ethyl)phenyl]formamido}pentanedioic acid
- (2R)-2-((4-(2-(2-Amino-4-oxo-3H,4H,7H-pyrrolo(2,3-d)pyrimidin-5-yl)ethyl)phenyl)formamido)pentanedioic acid
- (2R)-2-((4-(2-(2-Amino-4-oxo-4,7-dihydro-1H-pyrrolo(2,3-d)pyrimidin-5-yl)ethyl)benzoyl)amino)pentanedioic acid
- (4-(2-(2-Amino-4-oxo-4,7-dihydro-3H-pyrrolo(2,3-d)pyrimidin-5-yl)ethyl)benzoyl)-D-glutamic acid
- D-Glutamic acid, N-(4-(2-(2-amino-4,7-dihydro-4-oxo-3H-pyrrolo(2,3-d)pyrimidin-5-yl)ethyl)benzoyl)-
- PEMETREXED DISODIUM HEPTAHYDRATE IMPURITY E [EP IMPURITY]
- Pemetrexed EP Impurity E
- PEMETREXED DISODIUM HEPTAHYDRATE IMPURITY E (EP IMPURITY)
- C20H21N5O6
- N-[4-[2-(2-Amino-4,7-dihydro-4-oxo-3H-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl]benzoyl]-D-glutamic Acid Hydrate;
- SCHEMBL7969
- MLS006011486
- UNII-FG8Q47888S
- GTPL6837
- CHEBI:95021
- DTXSID70861342
- WBXPDJSOTKVWSJ-CYBMUJFWSA-N
- AKOS015895961
- SMR004703269
- CS-0164029
- NS00014353
- EN300-117237
- Pemetrexed disodium heptahydrate impurity E [EP]
- BRD-K55395145-001-01-7
- BRD-K55395145-304-01-5
- Z1501475005
- (2R)-2-[[4-[2-(2-amino-4-oxo-1,7-dihydropyrrolo[4,5-e]pyrimidin-5-yl)ethyl]benzoyl]amino]pentanedioic acid
- (2R)-2-[[4-[2-(2-amino-4-oxo-3,7-dihydropyrrolo[2,3-d]pyrimidin-5-yl)ethyl]benzoyl]amino]pentanedioic acid
- (2R)-2-{[4-(2-{2-amino-4-oxo-3H,4H,7H-pyrrolo[2,3-d]pyrimidin-5-yl}ethyl)phenyl]formamido}pentanedioicacid
- (R)-2-(4-(2-(2-amino-4-oxo-4,7-dihydro-1H-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl)benzamido)pentanedioic acid? (Pemetrexed Impurity pound(c)
- D-Glutamic acid, N-[4-[2-(2-amino-4,7-dihydro-4-oxo-3H-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl]benzoyl]-; D-Glutamic acid, N-[4-[2-(2-amino-4,7-dihydro-4-oxo-1H-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl]benzoyl]-; (2R)-2-[[4-[2-(2-Amino-4-oxo-1,7-dihydropyrrolo[2,3-d
- N-[4-[2-(2-Amino-4,7-dihydro-4-oxo-3H-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl]benzoyl]-D-glutamic Acid; (2R)-2-[[4-(2-[2-Amino-4-oxo-3H,4H,7H-pyrrolo[2,3-d]pyrimidin-5-yl]ethyl)phenyl]formamido]pentanedioic Acid; Pemetrexed EP Impurity E
321.089844 10097
450.132416 3277
343.06958 1221
163.054718 997
299.107239 916
- Cytoplasm
- Membrane


H315 (100%): Causes skin irritation [Warning Skin corrosion/irritation]
H319 (100%): Causes serious eye irritation [Warning Serious eye damage/eye irritation]
H341 (100%): Suspected of causing genetic defects [Warning Germ cell mutagenicity]
H360 (100%): May damage fertility or the unborn child [Danger Reproductive toxicity]
H373 (100%): May causes damage to organs through prolonged or repeated exposure [Warning Specific target organ toxicity, repeated exposure]
P203, P260, P264, P264+P265, P280, P302+P352, P305+P351+P338, P318, P319, P321, P332+P317, P337+P317, P362+P364, P405, and P501
(The corresponding statement to each P-code can be found at the GHS Classification page.)
Skin Irrit. 2 (100%)
Eye Irrit. 2 (100%)
Muta. 2 (100%)
Repr. 1B (100%)
STOT RE 2 (100%)
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=WBXPDJSOTKVWSJ-CYBMUJFWSA-N
- ChEBI(2R)-2-[[[4-[2-(2-amino-4-oxo-1,7-dihydropyrrolo[2,3-d]pyrimidin-5-yl)ethyl]phenyl]-oxomethyl]amino]pentanedioic acidhttps://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:95021
- ChemIDplusChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- EPA DSSTox(R)-Pemetrexedhttps://comptox.epa.gov/dashboard/DTXSID70861342CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-notice(2R)-2-[[4-[2-(2-amino-4-oxo-4,7-dihydro-1H-pyrrolo[2,3-d]pyrimidin-5-yl) ethyl]benzoyl] amino]pentanedioic acidhttps://echa.europa.eu/substance-information/-/substanceinfo/100.231.900(2R)-2-[[4-[2-(2-amino-4-oxo-4,7-dihydro-1H-pyrrolo[2,3-d]pyrimidin-5-yl) ethyl]benzoyl] amino]pentanedioic acid (EC: 805-167-0)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/238477
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingPEMETREXED, (R)-https://gsrs.ncats.nih.gov/ginas/app/beta/substances/FG8Q47888S
- Human Metabolome Database (HMDB)LICENSEHMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications.http://www.hmdb.ca/citingHMDB0014780_msms_374104https://hmdb.ca/metabolites/HMDB0014780#spectra
- IUPHAR/BPS Guide to PHARMACOLOGYLICENSEThe Guide to PHARMACOLOGY database is licensed under the Open Data Commons Open Database License (ODbL) https://opendatacommons.org/licenses/odbl/. Its contents are licensed under a Creative Commons Attribution-ShareAlike 4.0 International License (http://creativecommons.org/licenses/by-sa/4.0/)https://www.guidetopharmacology.org/about.jsp#licenseGuide to Pharmacology Target Classificationhttps://www.guidetopharmacology.org/targets.jsp
- Japan Chemical Substance Dictionary (Nikkaji)
- Springer Nature
- Wikidata(2R)-2-[[[4-[2-(2-amino-4-oxo-1,7-dihydropyrrolo[2,3-d]pyrimidin-5-yl)ethyl]phenyl]-oxomethyl]amino]pentanedioic acidhttps://www.wikidata.org/wiki/Q106029441
- PubChem
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/NORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 389242810https://pubchem.ncbi.nlm.nih.gov/substance/389242810