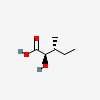(2R,3R)-2-hydroxy-3-methylpentanoic acid
- (2R,3R)-2-hydroxy-3-methylpentanoic acid
- 86540-81-0
- D-Isoleucic acid
- Pentanoic acid, 2-hydroxy-3-methyl-, (2R,3R)-
- isoleucic acid
- Create:2006-10-26
- Modify:2025-01-18
 2-Hydroxy-3-methylpentanoic acid (annotation moved to).
2-Hydroxy-3-methylpentanoic acid (annotation moved to).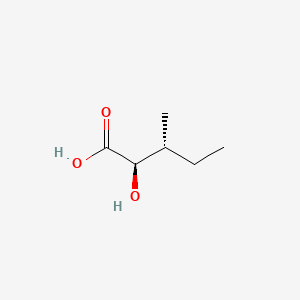
- (2R,3R)-2-hydroxy-3-methylpentanoic acid
- 86540-81-0
- D-Isoleucic acid
- Pentanoic acid, 2-hydroxy-3-methyl-, (2R,3R)-
- isoleucic acid
- 59653-35-9
- 2-hydroxy-3-methyl-pentanoic acid
- Isoleucic acid, (-)-
- 31PQT8YA0S
- Isoleucic acid, (+/-)-
- ZHK526L1KJ
- Rel-(2R,3R)-2-hydroxy-3-methylpentanoic acid
- 2R-hydroxy-3R-methyl-pentanoic acid
- 2-Hydroxy-3-methylvaleric acid, (2R,3R)-
- 2-Hydroxy-3-methylpentanoic acid, (2R,3R)-
- 2-Hydroxy-3-methylvalerate
- Pentanoic acid, 2-hydroxy-3-methyl-, (R*,R*)-
- Pentanoic acid, 2-hydroxy-3-methyl-, (2R,3R)-rel-
- alpha-hydroxy-beta-methylvaleric acid
- 2-hydroxy-3-methyl-Valeric acid
- A-hydroxy-b-methylvalerate
- UNII-31PQT8YA0S
- UNII-ZHK526L1KJ
- 2-hydroxy-3-methyl-Valerate
- A-hydroxy-b-methylvaleric acid
- 2-Hydroxy-3-methyl-pentanoate
- SCHEMBL1623516
- CHEBI:89228
- DTXSID601347302
- LMFA01050380
- HY-W074264
- (2R,3R)-2-hydroxy-3-methyl-pentanoate
- AS-88289
- DB-130840
- (2R,3R)-2-hydroxy-3-methyl-pentanoic acid
- CS-0108582
- D76826
- EN300-214785
- Q27161414
41.00193 100
43.01806 67.40
85.06566 100
131.07056 8.90
69.03373 7.20
43.01913 3.80
41.03895 3.40
41.00193 100
43.01806 67.40
41.03621 5.20
85.06573 100
131.07153 66.60
44.99745 6.20
85.10078 3.40
85.11488 3.10
 2-Hydroxy-3-methylpentanoic acid (annotation moved to)
2-Hydroxy-3-methylpentanoic acid (annotation moved to)- Extracellular
- Membrane

H315 (100%): Causes skin irritation [Warning Skin corrosion/irritation]
H319 (100%): Causes serious eye irritation [Warning Serious eye damage/eye irritation]
H335 (100%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation]
P261, P264, P264+P265, P271, P280, P302+P352, P304+P340, P305+P351+P338, P319, P321, P332+P317, P337+P317, P362+P364, P403+P233, P405, and P501
(The corresponding statement to each P-code can be found at the GHS Classification page.)
Aggregated GHS information provided per 2 reports by companies from 2 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies.
Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown.
Skin Irrit. 2 (100%)
Eye Irrit. 2A (100%)
STOT SE 3 (100%)
Silke Matysik, Caroline Ivanne Le Roy, Gerhard Liebisch, Sandrine Paule Claus. Metabolomics of fecal samples: A practical consideration. Trends in Food Science & Technology. Vol. 57, Part B, Nov. 2016, p.244-255: http://www.sciencedirect.com/science/article/pii/S0924224416301984
PubMed: 6422161, 12101068, 10508118, 10472531, 11978597, 19551947, 10234605, 23430924, 18088602
Peritoneal dialysis in maple-syrup-urine disease: Studies on branched-chain amino and keto acids. Eur J Pediatr (1980) 134: 57. https://doi.org/10.1007/BF00442404
MetaGene: Metabolic & Genetic Information Center (MIC: http://www.metagene.de)
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=RILPIWOPNGRASR-RFZPGFLSSA-N
- ChEBI(2R,3R)-2-hydroxy-3-methylpentanoic acidhttps://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:89228
- LOTUS - the natural products occurrence databaseLICENSEThe code for LOTUS is released under the GNU General Public License v3.0.https://lotus.nprod.net/(2R,3R)-2-hydroxy-3-methylpentanoic acidhttps://www.wikidata.org/wiki/Q27161414LOTUS Treehttps://lotus.naturalproducts.net/
- Yeast Metabolome Database (YMDB)2-Hydroxy-3-methylvaleratehttps://www.ymdb.ca/compounds/YMDB01571
- ChemIDplusIsoleucic acid, (+/-)-https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0059653359Isoleucic acid, (-)-https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0086540810ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- EPA DSSTox(2R,3R)-2-Hydroxy-3-methylpentanoic acidhttps://comptox.epa.gov/dashboard/DTXSID601347302CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-notice(2R,3R)-2-hydroxy-3-methylpentanoic acidhttps://echa.europa.eu/substance-information/-/substanceinfo/100.276.183(2S,3S)-2-hydroxy-3-methylpentanoic acidhttps://echa.europa.eu/substance-information/-/substanceinfo/100.362.240(2R,3R)-2-hydroxy-3-methylpentanoic acid (EC: 839-837-9)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/284998
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingISOLEUCIC ACID, (±)-https://gsrs.ncats.nih.gov/ginas/app/beta/substances/31PQT8YA0SISOLEUCIC ACID, (-)-https://gsrs.ncats.nih.gov/ginas/app/beta/substances/ZHK526L1KJ
- Human Metabolome Database (HMDB)LICENSEHMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications.http://www.hmdb.ca/citing2-Hydroxy-3-methylpentanoic acidhttp://www.hmdb.ca/metabolites/HMDB0000317HMDB0000317_msms_2244382https://hmdb.ca/metabolites/HMDB0000317#spectra
- FooDBLICENSEFooDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (FooDB) and the original publication.https://foodb.ca/about2-Hydroxy-3-methylpentanoic acidhttps://foodb.ca/compounds/FDB021944
- Japan Chemical Substance Dictionary (Nikkaji)
- LIPID MAPSLipid Classificationhttps://www.lipidmaps.org/
- Natural Product Activity and Species Source (NPASS)
- MassBank of North America (MoNA)LICENSEThe content of the MoNA database is licensed under CC BY 4.0.https://mona.fiehnlab.ucdavis.edu/documentation/license
- Metabolomics Workbench2-hydroxy-3-methyl-pentanoic acidhttps://www.metabolomicsworkbench.org/data/StructureData.php?RegNo=1497
- Wikidata(2R,3R)-2-hydroxy-3-methylpentanoic acidhttps://www.wikidata.org/wiki/Q27161414
- PubChem
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 389051795https://pubchem.ncbi.nlm.nih.gov/substance/389051795