Propionylcarnitine
- Propionylcarnitine
- 17298-37-2
- Propionyl carnitine
- 3-propanoyloxy-4-(trimethylazaniumyl)butanoate
- O-Propionylcarnitine
- Create:2005-06-24
- Modify:2025-01-18
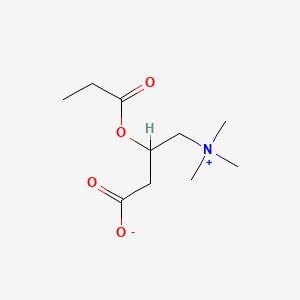
- L-propionylcarnitine
- propionyl carnitine
- propionylcarnitine
- propionylcarnitine, (+-)-isomer
- propionylcarnitine, (R)-isomer
- Propionylcarnitine
- 17298-37-2
- Propionyl carnitine
- 3-propanoyloxy-4-(trimethylazaniumyl)butanoate
- O-Propionylcarnitine
- 3-(propionyloxy)-4-(trimethylammonio)butanoate
- 3-(propanoyloxy)-4-(trimethylazaniumyl)butanoate
- CHEBI:28867
- Propionyl-Carnitine
- 3-Carboxy-N,N,N-trimethyl-2-(1-oxopropoxy)-1-propanaminium inner salt
- 1-Propanaminium, 3-carboxy-N,N,N-trimethyl-2-(1-oxopropoxy)-, inner salt
- HSDB 7589
- Acylcarnitine C3:0
- SCHEMBL156850
- CHEMBL2074916
- DTXSID20938255
- LMFA07070105
- AKOS030540155
- O-propanoylcarnitine (internal charge)
- HY-113092
- CS-0059547
- NS00015030
- C03017
- (3-Carboxy-2-propanoyloxypropyl)-trimethylazanium
- Q27103933
43.05414 100
71.08549 69.85
85.1011 58.21
202.10477 56.65
121.02828 46.96
73.02952 100
201.10072 17.20
59.01365 6.70
58.00587 6.20
216.0769 5.70
73.02949 100
216.0751 32.70
201.10031 21.40
59.01362 8.50
101.0246 7.20
159.064865 100
85.028046 95.82
144.101578 4.28
85.03434 4.24
159.081024 3.71
181.046921 100
198.939026 4.44
181.064438 4.37
165.800476 3.68
73.688545 3.64
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=UFAHZIUFPNSHSL-UHFFFAOYSA-N
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/Propionylcarnitinehttps://commonchemistry.cas.org/detail?cas_rn=17298-37-2
- ChemIDplusPropionyl-L-carnitinehttps://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0017298372ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- EPA DSSTox3-(Propanoyloxy)-4-(trimethylazaniumyl)butanoatehttps://comptox.epa.gov/dashboard/DTXSID20938255CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- Hazardous Substances Data Bank (HSDB)PROPIONYL-L-CARNITINEhttps://pubchem.ncbi.nlm.nih.gov/source/hsdb/7589
- ChEBIO-propanoylcarnitinehttps://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:28867
- LOTUS - the natural products occurrence databaseLICENSEThe code for LOTUS is released under the GNU General Public License v3.0.https://lotus.nprod.net/Propionylcarnitinehttps://www.wikidata.org/wiki/Q27103933LOTUS Treehttps://lotus.naturalproducts.net/
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.htmlChEMBL Protein Target Treehttps://www.ebi.ac.uk/chembl/g/#browse/targets
- Comparative Toxicogenomics Database (CTD)LICENSEIt is to be used only for research and educational purposes. Any reproduction or use for commercial purpose is prohibited without the prior express written permission of NC State University.http://ctdbase.org/about/legal.jsppropionylcarnitinehttps://ctdbase.org/detail.go?type=chem&acc=C003223
- FooDBLICENSEFooDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (FooDB) and the original publication.https://foodb.ca/aboutPropionylcarnitinehttps://foodb.ca/compounds/FDB022268
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.html
- LIPID MAPSLipid Classificationhttps://www.lipidmaps.org/
- Natural Product Activity and Species Source (NPASS)
- West Coast Metabolomics Center-UC DavisPropionylcarnitine
- MassBank of North America (MoNA)LICENSEThe content of the MoNA database is licensed under CC BY 4.0.https://mona.fiehnlab.ucdavis.edu/documentation/license
- Metabolomics WorkbenchPropionylcarnitinehttps://www.metabolomicsworkbench.org/data/StructureData.php?RegNo=76043
- NIST Mass Spectrometry Data CenterLICENSEData covered by the Standard Reference Data Act of 1968 as amended.https://www.nist.gov/srd/public-lawPropionylcarnitinehttp://www.nist.gov/srd/nist1a.cfm
- WikidataO-propanoylcarnitine (internal charge)https://www.wikidata.org/wiki/Q27103933
- PubChem
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlpropionylcarnitinehttps://www.ncbi.nlm.nih.gov/mesh/67003223Cardiotonic Agentshttps://www.ncbi.nlm.nih.gov/mesh/68002316Anti-Inflammatory Agents, Non-Steroidalhttps://www.ncbi.nlm.nih.gov/mesh/68000894
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/NORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 390285439https://pubchem.ncbi.nlm.nih.gov/substance/390285439
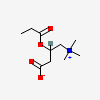

 CID 107739 (3-Carboxy-N,N,N-trimethyl-2-(propanoyloxy)propan-1-aminium)
CID 107739 (3-Carboxy-N,N,N-trimethyl-2-(propanoyloxy)propan-1-aminium)



