Pyruvate
PubChem CID
107735
Chemical Safety
Molecular Formula
Synonyms
- pyruvate
- 2-Oxopropanoate
- 57-60-3
- Methylglyoxylate
- Pyruvate ion
Molecular Weight
87.05 g/mol
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Parent Compound
Dates
- Create:2005-08-08
- Modify:2025-01-18
Description
Pyruvate is a 2-oxo monocarboxylic acid anion that is the conjugate base of pyruvic acid, arising from deprotonation of the carboxy group. It has a role as a fundamental metabolite and a cofactor. It is functionally related to a propionate. It is a conjugate base of a pyruvic acid.
Pyruvate is a 2-oxo monocarboxylic acid anion that is the conjugate base of pyruvic acid, which is an important metabolic product for energy-producing biochemical pathways. Pyruvate is the final product of glycolysis and, when hypoxic conditions arise, can be metabolized anaerobically to form lactate. This chemical is a precursor of both acetyl-coenzyme A and oxaloacetate, which are involved in the citric acid cycle. Additionally, oxaloacetate is a substrate in the gluconeogenesis pathway.
An intermediate compound in the metabolism of carbohydrates, proteins, and fats. In thiamine deficiency, its oxidation is retarded and it accumulates in the tissues, especially in nervous structures. (From Stedman, 26th ed)
Chemical Structure Depiction
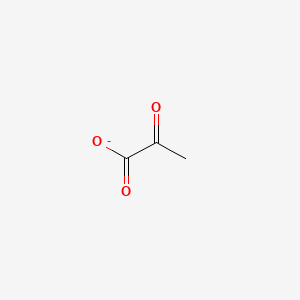
2-oxopropanoate
Computed by Lexichem TK 2.7.0 (PubChem release 2021.10.14)
InChI=1S/C3H4O3/c1-2(4)3(5)6/h1H3,(H,5,6)/p-1
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
LCTONWCANYUPML-UHFFFAOYSA-M
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
CC(=O)C(=O)[O-]
Computed by OEChem 2.3.0 (PubChem release 2024.12.12)
C3H3O3-
Computed by PubChem 2.2 (PubChem release 2021.10.14)
106-312-4
- Acid, Pyruvic
- Pyruvate
- Pyruvic Acid
- pyruvate
- 2-Oxopropanoate
- 57-60-3
- Methylglyoxylate
- Pyruvate ion
- HO43T60JMG
- 2-oxopropanoic acid, ion(1-)
- Natriumpyruvat
- Propanoic acid, 2-oxo-, ion(1-)
- Acid, Pyruvic
- 2-oxo-Propanoic acid
- CHEMBL181886
- 2hzl
- 2-oxidanylidenepropanoate
- UNII-HO43T60JMG
- CHEBI:15361
- PYRUVIC ACID, ION(1-)
- DTXSID50205604
- LCTONWCANYUPML-UHFFFAOYSA-M
- BDBM50159792
- DB-007695
- DB-053072
- A805655
- Q27089397
Property Name
Property Value
Reference
Property Name
Molecular Weight
Property Value
87.05 g/mol
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
XLogP3
Property Value
-0.6
Reference
Computed by XLogP3 3.0 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Donor Count
Property Value
0
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Acceptor Count
Property Value
3
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Rotatable Bond Count
Property Value
0
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Exact Mass
Property Value
87.008218953 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Monoisotopic Mass
Property Value
87.008218953 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Topological Polar Surface Area
Property Value
57.2 Ų
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Heavy Atom Count
Property Value
6
Reference
Computed by PubChem
Property Name
Formal Charge
Property Value
-1
Reference
Computed by PubChem
Property Name
Complexity
Property Value
78.5
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Isotope Atom Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Covalently-Bonded Unit Count
Property Value
1
Reference
Computed by PubChem
Property Name
Compound Is Canonicalized
Property Value
Yes
Reference
Computed by PubChem (release 2021.10.14)
MoNA ID
MS Category
Experimental
MS Type
Other
MS Level
MS2
Precursor Type
[M-H]-
Precursor m/z
87.01
Instrument
Orbitrap
Ionization Mode
negative
Top 5 Peaks
87.011528 100
54.004158 3.03
59.012932 2.49
51.818241 1.51
76.780098 1.48
Follow these links to do a live 2D search or do a live 3D search for this compound, sorted by annotation score. This section is deprecated (see here for details), but these live search links provide equivalent functionality to the table that was previously shown here.
Same Connectivity Count
Same Parent, Connectivity Count
Same Parent, Exact Count
Mixtures, Components, and Neutralized Forms Count
Similar Compounds (2D)
Similar Conformers (3D)
Same Count
PubMed Count
Pictogram(s)

Signal
Danger
GHS Hazard Statements
H314 (100%): Causes severe skin burns and eye damage [Danger Skin corrosion/irritation]
Precautionary Statement Codes
P260, P264, P280, P301+P330+P331, P302+P361+P354, P304+P340, P305+P354+P338, P316, P321, P363, P405, and P501
(The corresponding statement to each P-code can be found at the GHS Classification page.)
ECHA C&L Notifications Summary
The GHS information provided by 1 company from 1 notification to the ECHA C&L Inventory.
Skin Corr. 1B (100%)
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=LCTONWCANYUPML-UHFFFAOYSA-M
- BindingDBLICENSEAll data curated by BindingDB staff are provided under the Creative Commons Attribution 3.0 License (https://creativecommons.org/licenses/by/3.0/us/).https://www.bindingdb.org/rwd/bind/info.jsp
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/
- ChemIDplusChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- EPA DSSToxCompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-notice57-60-3https://echa.europa.eu
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- ChEBI
- NCI Thesaurus (NCIt)LICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuse
- ClinicalTrials.govLICENSEThe ClinicalTrials.gov data carry an international copyright outside the United States and its Territories or Possessions. Some ClinicalTrials.gov data may be subject to the copyright of third parties; you should consult these entities for any additional terms of use.https://clinicaltrials.gov/ct2/about-site/terms-conditions#Use
- Crystallography Open Database (COD)LICENSEAll data in the COD and the database itself are dedicated to the public domain and licensed under the CC0 License. Users of the data should acknowledge the original authors of the structural data.https://creativecommons.org/publicdomain/zero/1.0/
- Japan Chemical Substance Dictionary (Nikkaji)
- MassBank of North America (MoNA)LICENSEThe content of the MoNA database is licensed under CC BY 4.0.https://mona.fiehnlab.ucdavis.edu/documentation/license
- Natural Product Activity and Species Source (NPASS)
- Nature Chemical Biology
- Nature Chemistry
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/PyruvateNORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- PharmGKBLICENSEPharmGKB data are subject to the Creative Commons Attribution-ShareALike 4.0 license (https://creativecommons.org/licenses/by-sa/4.0/).https://www.pharmgkb.org/page/policies
- Rhea - Annotated Reactions DatabaseLICENSERhea has chosen to apply the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0/). This means that you are free to copy, distribute, display and make commercial use of the database in all legislations, provided you credit (cite) Rhea.https://www.rhea-db.org/help/license-disclaimer
- Springer Nature
- Thieme ChemistryLICENSEThe Thieme Chemistry contribution within PubChem is provided under a CC-BY-NC-ND 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc-nd/4.0/
- Wikidatapyruvate ionhttps://www.wikidata.org/wiki/Q27089397
- Wiley
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlPyruvic Acidhttps://www.ncbi.nlm.nih.gov/mesh/68019289
- PubChem
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- EPA Substance Registry ServicesEPA SRS List Classificationhttps://sor.epa.gov/sor_internet/registry/substreg/LandingPage.do
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 403720639https://pubchem.ncbi.nlm.nih.gov/substance/403720639
CONTENTS

 CID 1060 (Pyruvic Acid)
CID 1060 (Pyruvic Acid)