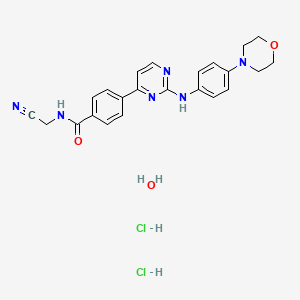
Momelotinib Hydrochloride Hydrate
- Momelotinib dihydrochloride monohydrate
- LDX8893L5D
- CYT-387 dihydrochloride monohydrate
- CYT-11387 dihydrochloride monohydrate
- UNII-LDX8893L5D
- Create:2015-12-28
- Modify:2025-01-18
- Momelotinib dihydrochloride monohydrate
- LDX8893L5D
- CYT-387 dihydrochloride monohydrate
- CYT-11387 dihydrochloride monohydrate
- UNII-LDX8893L5D
- Ojjaara
- MOMELOTINIB HYDROCHLORIDE HYDRATE
- Momelotinib hydrochloride hydrate (JAN)
- MOMELOTINIB HYDROCHLORIDE HYDRATE [JAN]
- 1841094-17-4
- Benzamide, N-(cyanomethyl)-4-(2-((4-(4-morpholinyl)phenyl)amino)-4-pyrimidinyl)-, hydrochloride, hydrate (1:2:1)
- Benzamide, N-(cyanomethyl)-4-[2-[[4-(4-morpholinyl)phenyl]amino]-4-pyrimidinyl]-, hydrochloride, hydrate (1:2:1)
- N-(Cyanomethyl)-4-(2-(4-(morpholin-4-yl)anilino)pyrimidin-4-yl)benzamide dihydrochloride monohydrate
- N-(Cyanomethyl)-4-{2-[4-(morpholin-4-yl)anilino]pyrimidin-4-yl}benzamide dihydrochloride monohydrate
- Ojjaara (TN)
- Omjjara (TN)
- D10889
Momelotinib dihydrochloride monohydrate is approved to treat:
• Myelofibrosis (a bone marrow disease) that is intermediate risk or high risk in adults with anemia, including the following types:
• Primary myelofibrosis.
• Post-polycythemia vera myelofibrosis.
• Post-essential thrombocythemia myelofibrosis.
Momelotinib dihydrochloride monohydrate is also being studied in the treatment of other types of cancer.


H302 (100%): Harmful if swallowed [Warning Acute toxicity, oral]
H373 (100%): May causes damage to organs through prolonged or repeated exposure [Warning Specific target organ toxicity, repeated exposure]
P260, P264, P270, P301+P317, P319, P330, and P501
(The corresponding statement to each P-code can be found at the GHS Classification page.)
Acute Tox. 4 (100%)
STOT RE 2 (100%)
◉ Summary of Use during Lactation
No information is available on the clinical use of momelotinib during breastfeeding. Because momelotinib is 91% bound to plasma proteins, the amount in milk is likely to be low. The manufacturer recommends that breastfeeding be discontinued during momelotinib therapy and for at least 1 week after the last dose.
◉ Effects in Breastfed Infants
Relevant published information was not found as of the revision date.
◉ Effects on Lactation and Breastmilk
Relevant published information was not found as of the revision date.
- ChemIDplusMomelotinib dihydrochloride monohydratehttps://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=1841094174ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-noticeN-(cyanomethyl)-4-[2-(4-morpholin-4-ylanilino)pyrimidin-4-yl]benzamide;hydrate;dihydrochloridehttps://echa.europa.euN-(cyanomethyl)-4-[2-(4-morpholin-4-ylanilino)pyrimidin-4-yl]benzamide;hydrate;dihydrochloride (EC: 991-915-2)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/379479
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingMomelotinib dihydrochloride monohydratehttps://gsrs.ncats.nih.gov/ginas/app/beta/substances/LDX8893L5D
- DailyMedMOMELOTINIB HYDROCHLORIDE HYDRATEhttps://dailymed.nlm.nih.gov/dailymed/search.cfm?labeltype=all&query=MOMELOTINIB+HYDROCHLORIDE+HYDRATE
- Drugs and Lactation Database (LactMed)
- Drugs@FDALICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- EU Clinical Trials Register
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.htmlTherapeutic category of drugs in Japanhttp://www.genome.jp/kegg-bin/get_htext?br08301.kegUSP drug classificationhttp://www.genome.jp/kegg-bin/get_htext?br08302.kegAnatomical Therapeutic Chemical (ATC) classificationhttp://www.genome.jp/kegg-bin/get_htext?br08303.kegTarget-based classification of drugshttp://www.genome.jp/kegg-bin/get_htext?br08310.keg
- National Drug Code (NDC) DirectoryLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingMOMELOTINIB HYDROCHLORIDE HYDRATEhttps://www.fda.gov/drugs/drug-approvals-and-databases/national-drug-code-directory
- NCI Cancer DrugsMomelotinib Dihydrochloride Monohydratehttps://www.cancer.gov/about-cancer/treatment/drugs/momelotinib-dihydrochloride-monohydrate
- NCI Thesaurus (NCIt)LICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuseNCI Thesaurushttps://ncit.nci.nih.gov
- NLM RxNorm TerminologyLICENSEThe RxNorm Terminology is created by the National Library of Medicine (NLM) and is in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from NLM. Credit to the U.S. National Library of Medicine as the source is appreciated but not required. The full RxNorm dataset requires a free license.https://www.nlm.nih.gov/research/umls/rxnorm/docs/termsofservice.htmlmomelotinib dihydrochloride monohydratehttps://rxnav.nlm.nih.gov/id/rxnorm/2665205
- PubChem
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
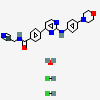

 CID 25062766 (Momelotinib)
CID 25062766 (Momelotinib) CID 313 (Hydrochloric Acid)
CID 313 (Hydrochloric Acid) CID 962 (Water)
CID 962 (Water)