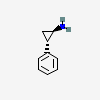Tranylcypromine
- tranylcypromine
- (1R,2S)-2-phenylcyclopropan-1-amine
- 3721-26-4
- (1R,2S)-2-phenylcyclopropanamine
- Parnate
- Create:2005-06-24
- Modify:2025-01-18
 Tranylcypromine Sulfate (has salt form);
Tranylcypromine Sulfate (has salt form);  Tranylcypromine hydrochloride (is active moiety of).
Tranylcypromine hydrochloride (is active moiety of).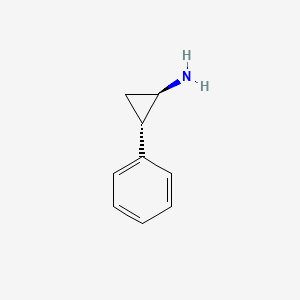
- Jatrosom
- Parnate
- Sulfate, Tranylcypromine
- trans 2 Phenylcyclopropylamine
- trans-2-Phenylcyclopropylamine
- Transamine
- Tranylcypromine
- Tranylcypromine Sulfate
- tranylcypromine
- (1R,2S)-2-phenylcyclopropan-1-amine
- 3721-26-4
- (1R,2S)-2-phenylcyclopropanamine
- Parnate
- 155-09-9
- 95-62-5
- Transamine
- Tranilcipromina
- Parnitene
- Tranylcyprominum
- trans-2-Phenylcyclopropylamine
- Cyclopropanamine, 2-phenyl-, (1R,2S)-rel-
- d-Tranylcypromine
- trans-2-phenyl-cyclopropylamine
- SKF Trans-385
- SKF 385
- trans-DL-2-Phenylcyclopropylamine
- (1R,2S)-tranylcypromine
- (+)-(R)-Tranylcypromine
- rel-Tranylcypromine
- NSC 80664
- Jatrosom
- UNII-3E3V44J4Z9
- CCRIS 3344
- l-Tranylcypromine
- HSDB 3404
- 3E3V44J4Z9
- EINECS 205-841-9
- trans-2-phenylcyclopropanamine
- BRN 3195803
- CHEMBL1179
- Tranylcyprominum [INN-Latin]
- rac-(1R,2S)-2-phenylcyclopropan-1-amine
- Tranilcipromina [INN-Spanish]
- DTXSID2023694
- (1R,2S)-2-phenyl-cyclopropylamine
- 3-12-00-02797 (Beilstein Handbook Reference)
- Racemic Tranylcypromine
- Cyclopropanamine, 2-phenyl-, (1S,2R)-
- trans-(-)-2-Phenylcyclopropanamine
- Cyclopropanamine, 2-phenyl-, trans-(.+/-.)-
- Cyclopropylamine, 2-phenyl-, trans-(.+/-.)-
- Tranylcyprominum (INN-Latin)
- Tranilcipromina (INN-Spanish)
- MFCD00865363
- NSC-80664
- 2-Phenyl-1-aminocyclopropane, trans-
- Parnate (Salt/Mix)
- (rel)-Tranylcypromine
- SKF-trans-385
- (1r,2s)-rel-2-phenylcyclopropanamine
- 2-Phenyl-cyclopropylamine
- Cyclopropylamine, 2-phenyl-, (1S-trans)-
- (.+/-.)-Tranylcypromine
- MFCD00001302
- TRANYLCYPROMINE [INN]
- Tranylcypromine [INN:BAN]
- trans-2-phenylcyclopropan-1-amine
- Tranylcypromin
- trans 2 Phenylcyclopropylamine
- trans amine
- trans-amine
- NSC80664
- Cyclopropanamine, 2-phenyl-, trans-(+-)-
- (+/-)-trans-2-phenylcyclopropylamine
- (.+/-.)-trans-2-Phenylcyclopropamine
- (.+/-.)-trans-2-Phenylcyclopropylamine
- (1R*,2S*)-2-phenylcyclopropan-1-amine
- GJZ
- BRN 2802413
- (1R,2S)-rel-2-phenylcyclopropan-1-amine
- Cyclopropylamine, 2-phenyl-, trans-(-)-
- Cyclopropanamine, 2-phenyl-, (1R-trans)-
- Cyclopropylamine, 2-phenyl-, trans-(+-)-
- Prestwick0_000173
- Prestwick1_000173
- Prestwick2_000173
- Lopac-P-8511
- CBChromo1_000132
- TRANYLCYPROMINE [MI]
- SCHEMBL34339
- TRANYLCYPROMINE [HSDB]
- SPBio_001986
- TRANYLCYPROMINE [VANDF]
- CHEBI:9652
- trans-2-phenyl cyclopropylamine
- (1R,2S)-trans-tranylcypromine
- TRANYLCYPROMINE [WHO-DD]
- (Trans)-2-phenylcyclopropanamine
- [trans-2-phenylcyclopropyl]amine
- CHEBI:94631
- cid_2723716
- N06AF04
- CHEBI:131510
- DTXCID501473942
- DTXSID201015773
- [(trans)-2-phenylcyclopropyl]amine
- SKF 385'
- (+-)-trans-2-phenylcyclopropylamine
- BDBM50240772
- NSC789036
- PDSP2_001079
- PDSP2_001080
- (1R-trans)-2-phenyl-cyclopropanamine
- AKOS006282586
- (1R,2S)-2-phenyl-1-cyclopropanamine
- cyclopropane, trans-1-amino-2-phenyl-
- NSC-789036
- (1R,2S)-(-)-2-phenylcyclopropylamine
- NCGC00015848-01
- NCGC00015848-07
- NCGC00015848-09
- (tranylcypromine)2-Phenyl-cyclopropylamine
- AC-15970
- BS-50045
- DA-77397
- CS-0099373
- trans-(1R,2S)-2-Phenylcyclopropan-1-amine
- EN300-197006
- EN300-253001
- BRD-K47029922-003-03-4
- BRD-K47029922-003-10-9
- BRD-K47029922-003-11-7
127.27 Ų [M+H]+ [CCS Type: TW; Method: calibrated with polyalanine and drug standards]
126.96 Ų [M+Na]+ [CCS Type: TW; Method: calibrated with polyalanine and drug standards]
Tranylcypromine Sulfate (has salt form)
Tranylcypromine hydrochloride (is active moiety of)
Use (kg; approx.) in Germany (2009): >25
Consumption (g per capita; approx.) in Germany (2009): 0.000305
Calculated removal (%): 75.3
IMAP assessments - Cyclopropanamine, 2-phenyl-, trans-(.+-.)-: Human health tier I assessment
IMAP assessments - Cyclopropanamine, 2-phenyl-, trans-(.+-.)-: Environment tier I assessment
Tranylcypromine, like most monoamine oxidase inhibitors, can cause transient serum aminotransferase elevations in a proportion of patients. These elevations are usually mild, asymptomatic and self-limited and do not require dose modification. Tranylcypromine has also been associated with rare cases of acute, clinically apparent liver injury. The few cases described have resembled those caused by other MAO inhibitors. The time to clinical onset is typically 1 to 4 months and the usual pattern of serum enzyme elevations is hepatocellular (Case 1), although cholestatic injury has also been described. Immunoallergic features (rash, fever, eosinophilia) are uncommon as is autoantibody formation.
Likelihood score: D (possible rare cause of clinically apparent liver injury).
◉ Summary of Use during Lactation
Because little information is available on the use of tranylcypromine during breastfeeding, an alternate drug may be preferred, especially while nursing a newborn or preterm infant.
◉ Effects in Breastfed Infants
A woman with severe depression took tranylcypromine 100 to 120 mg daily, as well as pimozide, diazepam and alprazolam during pregnancy and postpartum. She breastfed her infant until about 2 weeks postpartum when the infant developed abdominal distension and feeding intolerance. The symptoms resolved on discontinuation of breastfeeding.
◉ Effects on Lactation and Breastmilk
Nine subjects were treated with an average dose of 29 mg daily (range 10 to 40 mg daily) of oral tranylcypromine for an average of 16 days. Serum prolactin levels increased by 3 mcg/L.The clinical relevance of these findings in nursing mothers is not known. The prolactin level in a mother with established lactation may not affect her ability to breastfeed.
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=AELCINSCMGFISI-DTWKUNHWSA-N
- Avoid alcohol. Ingesting alcohol may increase the CNS depressant effects of tranylcypromine.
- Avoid St. John's Wort. Administering tranylcypromine with St. John's Wort may increase the risk of serotonin syndrome.
- Avoid tyramine-containing foods and supplements. Tyramine-containing foods and beverages can cause a sudden elevation in blood pressure or a hypertensive crisis. Foods that contain tyramine include aged cheese, ripe bananas, red wine, some alcoholic beverages (beer), cured food, pickled food, and fava beans.
- Australian Industrial Chemicals Introduction Scheme (AICIS)Cyclopropanamine, 2-phenyl-, trans-(.+-.)-https://services.industrialchemicals.gov.au/search-assessments/
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/(-)-Tranylcyprominehttps://commonchemistry.cas.org/detail?cas_rn=3721-26-4Tranylcyprominehttps://commonchemistry.cas.org/detail?cas_rn=155-09-9
- ChemIDplusTranylcypromine [INN:BAN]https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000155099Cyclopropylamine, 2-phenyl-, trans-(-)-https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0003721264Cyclopropylamine, 2-phenyl-, (1S-trans)-https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0003721286ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- DrugBankLICENSECreative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode)https://www.drugbank.ca/legal/terms_of_useTranylcyprominehttps://www.drugbank.ca/drugs/DB00752
- EPA DSSToxTranylcyprominehttps://comptox.epa.gov/dashboard/DTXSID2023694(1R,2S)-Tranylcyprominehttps://comptox.epa.gov/dashboard/DTXSID201015773CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-notice
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- Hazardous Substances Data Bank (HSDB)TRANYLCYPROMINEhttps://pubchem.ncbi.nlm.nih.gov/source/hsdb/3404
- New Zealand Environmental Protection Authority (EPA)LICENSEThis work is licensed under the Creative Commons Attribution-ShareAlike 4.0 International licence.https://www.epa.govt.nz/about-this-site/general-copyright-statement/
- CCSbaseCCSbase Classificationhttps://ccsbase.net/
- ChEBI(1R,2S)-tranylcyprominehttps://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:131510
- FDA Pharm ClassesLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingFDA Pharmacological Classificationhttps://www.fda.gov/ForIndustry/DataStandards/StructuredProductLabeling/ucm162549.htm
- LiverTox
- NCI Thesaurus (NCIt)LICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuseNCI Thesaurushttps://ncit.nci.nih.gov
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.htmlChEMBL Protein Target Treehttps://www.ebi.ac.uk/chembl/g/#browse/targets
- ClinicalTrials.govLICENSEThe ClinicalTrials.gov data carry an international copyright outside the United States and its Territories or Possessions. Some ClinicalTrials.gov data may be subject to the copyright of third parties; you should consult these entities for any additional terms of use.https://clinicaltrials.gov/ct2/about-site/terms-conditions#Use
- Comparative Toxicogenomics Database (CTD)LICENSEIt is to be used only for research and educational purposes. Any reproduction or use for commercial purpose is prohibited without the prior express written permission of NC State University.http://ctdbase.org/about/legal.jspTranylcyprominehttps://ctdbase.org/detail.go?type=chem&acc=D014191
- Drug Gene Interaction database (DGIdb)LICENSEThe data used in DGIdb is all open access and where possible made available as raw data dumps in the downloads section.http://www.dgidb.org/downloadsTRANYLCYPROMINEhttps://www.dgidb.org/drugs/rxcui:10734
- Therapeutic Target Database (TTD)Tranylcyprominehttps://idrblab.net/ttd/data/drug/details/D0H0HJ
- DailyMed
- Drugs and Lactation Database (LactMed)Tranylcyprominehttps://www.ncbi.nlm.nih.gov/books/n/lactmed/LM311/
- Drugs@FDALICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/(+)-tranylcypromine | ParnateNORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- EU Clinical Trials Register
- National Drug Code (NDC) DirectoryLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- IUPAC Digitized pKa Datasetcyclopropane, trans-1-amino-2-phenyl-https://github.com/IUPAC/Dissociation-Constants
- Japan Chemical Substance Dictionary (Nikkaji)
- Metabolomics Workbench
- NIST Mass Spectrometry Data CenterLICENSEData covered by the Standard Reference Data Act of 1968 as amended.https://www.nist.gov/srd/public-lawTranylcyprominehttp://www.nist.gov/srd/nist1a.cfm
- NLM RxNorm TerminologyLICENSEThe RxNorm Terminology is created by the National Library of Medicine (NLM) and is in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from NLM. Credit to the U.S. National Library of Medicine as the source is appreciated but not required. The full RxNorm dataset requires a free license.https://www.nlm.nih.gov/research/umls/rxnorm/docs/termsofservice.htmltranylcyprominehttps://rxnav.nlm.nih.gov/id/rxnorm/10734
- PharosLICENSEData accessed from Pharos and TCRD is publicly available from the primary sources listed above. Please respect their individual licenses regarding proper use and redistribution.https://pharos.nih.gov/abouttranylcyprominehttps://pharos.nih.gov/ligands/LM17WG8J4MHTtranylcyprominehttps://pharos.nih.gov/ligands/LM1GZFVJ9TM4Tranylcyprominehttps://pharos.nih.gov/ligands/VSBCH4YBRFLH
- Protein Data Bank in Europe (PDBe)
- RCSB Protein Data Bank (RCSB PDB)LICENSEData files contained in the PDB archive (ftp://ftp.wwpdb.org) are free of all copyright restrictions and made fully and freely available for both non-commercial and commercial use. Users of the data should attribute the original authors of that structural data.https://www.rcsb.org/pages/policies
- SpectraBase(1R,2S)-2-phenyl-1-cyclopropanaminehttps://spectrabase.com/spectrum/ElD2k2JwsfR
- Springer Nature
- Thieme ChemistryLICENSEThe Thieme Chemistry contribution within PubChem is provided under a CC-BY-NC-ND 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc-nd/4.0/
- WHO Anatomical Therapeutic Chemical (ATC) ClassificationLICENSEUse of all or parts of the material requires reference to the WHO Collaborating Centre for Drug Statistics Methodology. Copying and distribution for commercial purposes is not allowed. Changing or manipulating the material is not allowed.https://www.whocc.no/copyright_disclaimer/Tranylcyprominehttps://www.whocc.no/atc_ddd_index/?code=N06AF04
- Wikidatatrans-(+)-tranylcyprominehttps://www.wikidata.org/wiki/Q100429273
- WikipediaTranylcyprominehttps://en.wikipedia.org/wiki/Tranylcypromine
- Wiley
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlTranylcyprominehttps://www.ncbi.nlm.nih.gov/mesh/68014191Antidepressive Agentshttps://www.ncbi.nlm.nih.gov/mesh/68000928Monoamine Oxidase Inhibitorshttps://www.ncbi.nlm.nih.gov/mesh/68008996Anti-Anxiety Agentshttps://www.ncbi.nlm.nih.gov/mesh/68014151
- PubChem
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 403383626https://pubchem.ncbi.nlm.nih.gov/substance/403383626
- NCBI