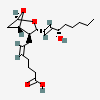
Thromboxane A2
PubChem CID
5280497
Molecular Formula
Synonyms
- thromboxane A2
- TXA2
- 57576-52-0
- TXA-2
- Rabbit aorta contracting substance
Molecular Weight
352.5 g/mol
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Dates
- Create:2004-09-16
- Modify:2025-01-04
Description
Thromboxane A2 is a thromboxane which is produced by activated platelets and has prothrombotic properties: it stimulates activation of new platelets as well as increases platelet aggregation. It has a role as a mouse metabolite. It is an epoxy monocarboxylic acid and a thromboxanes A. It is a conjugate acid of a thromboxane A2(1-).
thromboxane A2 has been reported in Streptomyces, Hypericum perforatum, and other organisms with data available.
Thromboxane A2 is an eicosanoid and short-lived intermediate product between prostaglandin endoperoxides and thromboxane B2, with prothrombotic and vasoconstrictive activities. Thromboxane A (TXA2) binds to its cognate receptor thromboxane A2 receptor (TBXA2R) and stimulates increased expression of glycoprotein IIb/IIIa (GPIIb/IIIa) on platelet membranes, which induces platelet activation and aggregation. Circulating fibrinogen binds to GPIIb/IIIa on platelet surfaces, which promotes clotting. TXA2 also binds to TBXA2R expressed by glomerular cells, which induces signaling pathways that promote vasoconstriction.
Chemical Structure Depiction

(Z)-7-[(1S,3R,4S,5S)-3-[(E,3S)-3-hydroxyoct-1-enyl]-2,6-dioxabicyclo[3.1.1]heptan-4-yl]hept-5-enoic acid
Computed by Lexichem TK 2.7.0 (PubChem release 2021.10.14)
InChI=1S/C20H32O5/c1-2-3-6-9-15(21)12-13-17-16(18-14-20(24-17)25-18)10-7-4-5-8-11-19(22)23/h4,7,12-13,15-18,20-21H,2-3,5-6,8-11,14H2,1H3,(H,22,23)/b7-4-,13-12+/t15-,16+,17+,18-,20+/m0/s1
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
DSNBHJFQCNUKMA-SCKDECHMSA-N
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
CCCCC[C@@H](/C=C/[C@@H]1[C@H]([C@@H]2C[C@@H](O2)O1)C/C=C\CCCC(=O)O)O
Computed by OEChem 2.3.0 (PubChem release 2024.12.12)
C20H32O5
Computed by PubChem 2.2 (PubChem release 2021.10.14)
- A2, Thromboxane
- Rabbit Aorta Contracting Substance
- Thromboxane A2
- thromboxane A2
- TXA2
- 57576-52-0
- TXA-2
- Rabbit aorta contracting substance
- UNII-4C2A5G825S
- 4C2A5G825S
- 9S,11S-epoxy,15S-hydroxy-thromboxa-5Z,13E-dien-1-oic acid
- (Z)-7-[(1S,3R,4S,5S)-3-[(E,3S)-3-hydroxyoct-1-enyl]-2,6-dioxabicyclo[3.1.1]heptan-4-yl]hept-5-enoic acid
- THROMBOXANE A2 [MI]
- Thromboxa-5,13-dien-1-oic acid, 9,11-epoxy-15-hydroxy-, (5Z,9alpha,11alpha,13E,15S)-
- CHEBI:15627
- (5Z,13E)-(15S)-9alpha,11alpha-Epoxy-15-hydroxythromboxa-5,13-dienoate
- (5Z,9alpha,11alpha,13E,15S)-9,11-Epoxy-15-hydroxythromboxa-5,13-dien-1-oic acid
- Benz(a)anthracene-1,7,12(2H)-trione, 3,4-dihydro-3,6,8-trihydroxy-3-methyl-, (-)-
- 5-Heptenoic acid, 7-(3-(3-hydroxy-1-octenyl)-2,6-dioxabicyclo(3.1.1)hept-4-yl)-, (1S-(1alpha,3alpha,3R*),4beta(Z),5alpha)-
- A2, Thromboxane
- (5Z,13E,15S)-9alpha,11alpha-epoxy-15-hydroxythromboxa-5,13-dien-1-oic acid
- (5Z,9.ALPHA.,11.ALPHA.,13E,15S)-9,11-EPOXY-15-HYDROXYTHROMBOXA-5,13-DIEN-1-OIC ACID
- (Z)-7-[(1S,2S,3R,5S)-3-[(E,3S)-3-hydroxyoct-1-enyl]-4,7-dioxabicyclo[3.1.1]heptan-2-yl]hept-5-enoic acid
- 5-HEPTENOIC ACID, 7-((1S,3R,4S,5S)-3-((1E,3S)-3-HYDROXY-1-OCTEN-1-YL)-2,6-DIOXABICYCLO(3.1.1)HEPT-4-YL)-, (5Z)-
- RCS
- (Z)-7-((1S,2S,3R,5S)-3-((E,3S)-3-hydroxyoct-1-enyl)-4,7-dioxabicyclo(3.1.1)heptan-2-yl)hept-5-enoic acid
- (Z)-7-((1S,3R,4S,5S)-3-((E,3S)-3-hydroxyoct-1-enyl)-2,6-dioxabicyclo(3.1.1)heptan-4-yl)hept-5-enoic acid
- SCHEMBL34165
- GTPL4482
- CHEMBL5283529
- BDBM82229
- 7-[3-(3-HYDROXYOCT-1-ENYL)-4,6-DIOXABICYCLO[3.1.1]HEPT-2-YL]HEPT-5-ENOIC ACID
- DSNBHJFQCNUKMA-SCKDECHMSA-N
- DTXSID201317452
- CMC_9718
- LMFA03030001
- AKOS040754197
- DA-68172
- CAS_57576-52-0
- HY-113350
- C02198
- Q774909
- 9S,11S-epoxy,15S-hydroxy-thromboxa-5Z,13E-dien-1-oate
- (5Z,13E)-(15S)-9,11-epoxy-15-hydroxythromba-5,13-dienoate
- (5Z,13E)-(15S)-9,11-epoxy-15-hydroxythromba-5,13-dienoic acid
- (5Z,13E)-(15S)-9a,11a-epoxy-15-Hydroxythromboxa-5,13-dienoate
- (5Z,13E)-(15S)-9a,11a-epoxy-15-Hydroxythromboxa-5,13-dienoic acid
- 2,6-Dioxabicyclo[3.1.1]heptane, thromboxa-5,13-dien-1-oic acid deriv.
- (5Z, 9alpha,11 alpha,13E,15S)-9,11-epoxy-15-hydroxythromboxa-5,13-dien-1-oic acid
- (5Z,13E)-(15S)-9-alpha,11-alpha-epoxy-15-hydroxythromboxa-5,13-dienoate
- (5Z,13E)-(15S)-9-alpha,11-alpha-Epoxy-15-hydroxythromboxa-5,13-dienoic acid
- (5Z,13E)-(15S)-9alpha,11alpha-epoxy-15-hydroxythromboxa-5,13-dienoic acid
- (5Z,9alpha,11alpha,13E,15S)-9,11-epoxy-15-hydroxy-Thromboxa-5,13-dien-1-oate
- (5Z,9alpha,11alpha,13E,15S)-9,11-epoxy-15-hydroxy-Thromboxa-5,13-dien-1-oic acid
- (5Z,9alpha,11alpha,13E,15S)-9,11-Epoxy-15-hydroxythromboxa-5,13-dien-1-oate
- (1S-(1alpha,3alpha,3R*),4beta(Z),5alpha)-7-(3-(3-hydroxy-1-octenyl)-2,6-dioxabicyclo(3.1.1)hept-4-yl)-5-Heptenoate
- (1S-(1alpha,3alpha,3R*),4beta(Z),5alpha)-7-(3-(3-hydroxy-1-octenyl)-2,6-dioxabicyclo(3.1.1)hept-4-yl)-5-Heptenoic acid
- (5Z)-7-[(1S,3R,4S,5S)-3-[(1E,3S)-3-hydroxy-1-octenyl]-2,6-dioxabicyclo[3.1.1]hept-4-yl]-5-Heptenoate
- (5Z)-7-[(1S,3R,4S,5S)-3-[(1E,3S)-3-hydroxy-1-octenyl]-2,6-dioxabicyclo[3.1.1]hept-4-yl]-5-Heptenoic acid
- (5Z,9alpha,11alpha,13E,15S)-9,11-epoxy-15-hydroxythromboxa-5,13-dien-1-oic acid, [1S-[1alpha,3alpha-(1E,3R*)4beta(Z),5alpha]]-7-[3-(3-hydroxy-1-octenyl)-2,6-dioxabicyclo[3.1.1]hept-4-yl]-5-heptenoic a
- 5-Heptenoic acid, 7-[(1S,3R,4S,5S)-3-[(1E,3S)-3-hydroxy-1-octenyl]-2,6-dioxabicyclo[3.1.1]hept-4-yl]-, (5Z)-
- 5-Heptenoic acid, 7-[3-(3-hydroxy-1-octenyl)-2,6-dioxabicyclo[3.1.1]hept-4-yl]-, [1S-[1alpha,3alpha(1E,3R*),4beta(Z),5alpha]]-
- 7-[3-(3-hydroxy-1-octenyl)-2,6-dioxabicyclo[3.1.1]hept-4-yl]-[1S-[1alpha,3alpha(1E,3R*),4beta(Z),5alpha]]-5-Heptenoate
- 7-[3-(3-hydroxy-1-octenyl)-2,6-dioxabicyclo[3.1.1]hept-4-yl]-[1S-[1alpha,3alpha(1E,3R*),4beta(Z),5alpha]]-5-Heptenoic acid
Property Name
Property Value
Reference
Property Name
Molecular Weight
Property Value
352.5 g/mol
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
XLogP3-AA
Property Value
3.4
Reference
Computed by XLogP3 3.0 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Donor Count
Property Value
2
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Acceptor Count
Property Value
5
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Rotatable Bond Count
Property Value
12
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Exact Mass
Property Value
352.22497412 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Monoisotopic Mass
Property Value
352.22497412 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Topological Polar Surface Area
Property Value
76 Ų
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Heavy Atom Count
Property Value
25
Reference
Computed by PubChem
Property Name
Formal Charge
Property Value
0
Reference
Computed by PubChem
Property Name
Complexity
Property Value
459
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Isotope Atom Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Atom Stereocenter Count
Property Value
5
Reference
Computed by PubChem
Property Name
Undefined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Bond Stereocenter Count
Property Value
2
Reference
Computed by PubChem
Property Name
Undefined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Covalently-Bonded Unit Count
Property Value
1
Reference
Computed by PubChem
Property Name
Compound Is Canonicalized
Property Value
Yes
Reference
Computed by PubChem (release 2021.10.14)
Solid
Fatty Acyls [FA] -> Eicosanoids [FA03] -> Thromboxanes [FA0303]
Follow these links to do a live 2D search or do a live 3D search for this compound, sorted by annotation score. This section is deprecated (see here for details), but these live search links provide equivalent functionality to the table that was previously shown here.
Same Connectivity Count
Same Parent, Connectivity Count
Same Parent, Exact Count
Mixtures, Components, and Neutralized Forms Count
Similar Compounds (2D)
Similar Conformers (3D)
- Platelet
- Smooth Muscle
- Cytoplasm
- Endoplasmic reticulum
- Extracellular
- Membrane
- Acetaminophen Action Pathway
- Acetylsalicylic Acid Action Pathway
- Antipyrine Action Pathway
- Antrafenine Action Pathway
- Arachidonic Acid Metabolism
- Bromfenac Action Pathway
- Carprofen Action Pathway
- Celecoxib Action Pathway
- Diclofenac Action Pathway
- Diflunisal Action Pathway
- Total 41 pathways, visit the HMDB page for details
Disease
References
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=DSNBHJFQCNUKMA-SCKDECHMSA-N
Zebrafish Pathway Metabolite MetFrag Local CSV (Beta) | DOI:10.5281/zenodo.3457553
The LOTUS Initiative for Open Natural Products Research: frozen dataset union wikidata (with metadata) | DOI:10.5281/zenodo.5794106
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/Thromboxane A2https://commonchemistry.cas.org/detail?cas_rn=57576-52-0
- ChemIDplusChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- EPA DSSToxThromboxane A2https://comptox.epa.gov/dashboard/DTXSID201317452CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- Human Metabolome Database (HMDB)LICENSEHMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications.http://www.hmdb.ca/citingThromboxane A2http://www.hmdb.ca/metabolites/HMDB0001452
- ChEBI
- LOTUS - the natural products occurrence databaseLICENSEThe code for LOTUS is released under the GNU General Public License v3.0.https://lotus.nprod.net/thromboxane A2https://www.wikidata.org/wiki/Q774909LOTUS Treehttps://lotus.naturalproducts.net/
- NCI Thesaurus (NCIt)LICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuseNCI Thesaurushttps://ncit.nci.nih.gov
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.html
- Comparative Toxicogenomics Database (CTD)LICENSEIt is to be used only for research and educational purposes. Any reproduction or use for commercial purpose is prohibited without the prior express written permission of NC State University.http://ctdbase.org/about/legal.jspThromboxane A2https://ctdbase.org/detail.go?type=chem&acc=D013928
- ECI Group, LCSB, University of LuxembourgThromboxane A2
- Natural Product Activity and Species Source (NPASS)
- IUPHAR/BPS Guide to PHARMACOLOGYLICENSEThe Guide to PHARMACOLOGY database is licensed under the Open Data Commons Open Database License (ODbL) https://opendatacommons.org/licenses/odbl/. Its contents are licensed under a Creative Commons Attribution-ShareAlike 4.0 International License (http://creativecommons.org/licenses/by-sa/4.0/)https://www.guidetopharmacology.org/about.jsp#licensethromboxane A<sub>2</sub>https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4482
- Japan Chemical Substance Dictionary (Nikkaji)
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.htmlCompounds with biological roleshttp://www.genome.jp/kegg-bin/get_htext?br08001.keg
- LIPID MAPSLipid Classificationhttps://www.lipidmaps.org/
- Metabolomics Workbench
- PharmGKBLICENSEPharmGKB data are subject to the Creative Commons Attribution-ShareALike 4.0 license (https://creativecommons.org/licenses/by-sa/4.0/).https://www.pharmgkb.org/page/policiesthromboxane A2https://www.pharmgkb.org/chemical/PA166178672
- Thieme ChemistryLICENSEThe Thieme Chemistry contribution within PubChem is provided under a CC-BY-NC-ND 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc-nd/4.0/
- Wikidatathromboxane a2https://www.wikidata.org/wiki/Q774909
- WikipediaBourgeonalhttps://en.wikipedia.org/wiki/BourgeonalThromboxane A2https://en.wikipedia.org/wiki/Thromboxane_A2
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlThromboxane A2https://www.ncbi.nlm.nih.gov/mesh/68013928
- PubChem
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 390414117https://pubchem.ncbi.nlm.nih.gov/substance/390414117
- NCBI
CONTENTS