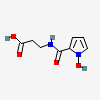
Surugapyrrole B
PubChem CID
70678742
Molecular Formula
Synonyms
- surugapyrrole B
- CHEBI:66535
- N-[(1-hydroxy-1H-pyrrol-2-yl)carbonyl]-beta-alanine
- 3-{[(1-hydroxy-1H-pyrrol-2-yl)carbonyl]amino}propanoic acid
- 1178873-34-1
Molecular Weight
198.18 g/mol
Computed by PubChem 2.1 (PubChem release 2021.05.07)
Dates
- Create:2012-12-12
- Modify:2025-01-18
Description
Surugapyrrole B is a pyrrolecarboxamide obtained by the formal condensation of 1-hydroxy-1H-pyrrole-2-carboxylic acid with the amino group of 3-aminopropanoic acid. It is isolated from the culture broth of Streptomyces sp.USF-6280 and exhibits DPPH radical scavenging activity. It has a role as a metabolite and a radical scavenger. It is a monocarboxylic acid, a N-hydroxypyrrole, a pyrrolecarboxamide and a beta-alanine derivative.
surugapyrrole B has been reported in Streptomyces with data available.
Chemical Structure Depiction
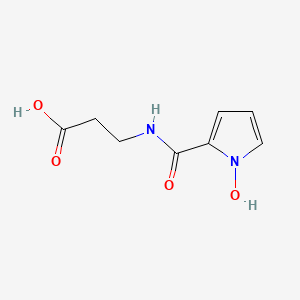
3-[(1-hydroxypyrrole-2-carbonyl)amino]propanoic acid
Computed by Lexichem TK 2.7.0 (PubChem release 2021.05.07)
InChI=1S/C8H10N2O4/c11-7(12)3-4-9-8(13)6-2-1-5-10(6)14/h1-2,5,14H,3-4H2,(H,9,13)(H,11,12)
Computed by InChI 1.0.6 (PubChem release 2021.05.07)
SYKRZHVRQXPZOX-UHFFFAOYSA-N
Computed by InChI 1.0.6 (PubChem release 2021.05.07)
C1=CN(C(=C1)C(=O)NCCC(=O)O)O
Computed by OEChem 2.3.0 (PubChem release 2024.12.12)
C8H10N2O4
Computed by PubChem 2.1 (PubChem release 2021.05.07)
- surugapyrrole B
- CHEBI:66535
- N-[(1-hydroxy-1H-pyrrol-2-yl)carbonyl]-beta-alanine
- 3-{[(1-hydroxy-1H-pyrrol-2-yl)carbonyl]amino}propanoic acid
- 1178873-34-1
- 3-((1-hydroxypyrrole-2-carbonyl)amino)propanoic acid
- 3-[(1-hydroxypyrrole-2-carbonyl)amino]propanoic acid
- N-((1-Hydroxy-1H-pyrrol-2-yl)carbonyl)-beta-alanine
- 3-(((1-hydroxy-1H-pyrrol-2-yl)carbonyl)amino)propanoate
- 3-{[(1-hydroxy-1H-pyrrol-2-yl)carbonyl]amino}propanoate
- 3-(((1-hydroxy-1H-pyrrol-2-yl)carbonyl)amino)propanoic acid
- DTXSID101239346
- Q27135144
- N-[(1-Hydroxy-1H-pyrrol-2-yl)carbonyl]-I(2)-alanine
Property Name
Property Value
Reference
Property Name
Molecular Weight
Property Value
198.18 g/mol
Reference
Computed by PubChem 2.1 (PubChem release 2021.05.07)
Property Name
XLogP3-AA
Property Value
0.4
Reference
Computed by XLogP3 3.0 (PubChem release 2021.05.07)
Property Name
Hydrogen Bond Donor Count
Property Value
3
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.05.07)
Property Name
Hydrogen Bond Acceptor Count
Property Value
4
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.05.07)
Property Name
Rotatable Bond Count
Property Value
4
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.05.07)
Property Name
Exact Mass
Property Value
198.06405680 Da
Reference
Computed by PubChem 2.1 (PubChem release 2021.05.07)
Property Name
Monoisotopic Mass
Property Value
198.06405680 Da
Reference
Computed by PubChem 2.1 (PubChem release 2021.05.07)
Property Name
Topological Polar Surface Area
Property Value
91.6 Ų
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.05.07)
Property Name
Heavy Atom Count
Property Value
14
Reference
Computed by PubChem
Property Name
Formal Charge
Property Value
0
Reference
Computed by PubChem
Property Name
Complexity
Property Value
231
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.05.07)
Property Name
Isotope Atom Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Covalently-Bonded Unit Count
Property Value
1
Reference
Computed by PubChem
Property Name
Compound Is Canonicalized
Property Value
Yes
Reference
Computed by PubChem (release 2012.11.26)
Follow these links to do a live 2D search or do a live 3D search for this compound, sorted by annotation score. This section is deprecated (see here for details), but these live search links provide equivalent functionality to the table that was previously shown here.
Similar Compounds (2D)
Similar Conformers (3D)
Same Count
The LOTUS Initiative for Open Natural Products Research: frozen dataset union wikidata (with metadata) | DOI:10.5281/zenodo.5794106
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/N-[(1-Hydroxy-1H-pyrrol-2-yl)carbonyl]-β-alaninehttps://commonchemistry.cas.org/detail?cas_rn=1178873-34-1
- EPA DSSToxN-[(1-Hydroxy-1H-pyrrol-2-yl)carbonyl]-β-alaninehttps://comptox.epa.gov/dashboard/DTXSID101239346CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- ChEBISurugapyrrole Bhttps://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:66535
- LOTUS - the natural products occurrence databaseLICENSEThe code for LOTUS is released under the GNU General Public License v3.0.https://lotus.nprod.net/surugapyrrole Bhttps://www.wikidata.org/wiki/Q27135144LOTUS Treehttps://lotus.naturalproducts.net/
- Japan Chemical Substance Dictionary (Nikkaji)
- Natural Product Activity and Species Source (NPASS)
- Metabolomics Workbench
- Wikidatasurugapyrrole Bhttps://www.wikidata.org/wiki/Q27135144
- PubChem
- The Natural Products AtlasLICENSEThe Natural Products Atlas is licensed under a Creative Commons Attribution 4.0 International License.https://www.npatlas.org/termsThe Natural Products Atlas Classificationhttps://www.npatlas.org/
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
CONTENTS