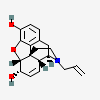Nalorphine
PubChem CID
5284595
Molecular Formula
Synonyms
- Nalorphine
- Allorphine
- Anthorphine
- Antorphine
- Nalorphinium
Molecular Weight
311.4 g/mol
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Dates
- Create:2005-06-24
- Modify:2025-01-11
Description
Nalorphine is a morphinane alkaloid.
Nalorphine is a mixed opioid agonist–antagonist. It acts at two opioid receptors—at the mu receptor it has antagonistic effects, and at the kappa receptors it exerts high-efficacy agonistic characteristics. It is used to reverse opioid overdose and (starting in the 1950s) in a challenge test to determine opioid dependence.
Nalorphine has been reported in Papaver somniferum with data available.
Chemical Structure Depiction
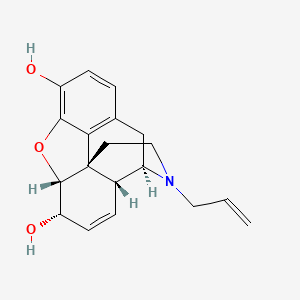
(4R,4aR,7S,7aR,12bS)-3-prop-2-enyl-2,4,4a,7,7a,13-hexahydro-1H-4,12-methanobenzofuro[3,2-e]isoquinoline-7,9-diol
Computed by Lexichem TK 2.7.0 (PubChem release 2021.10.14)
InChI=1S/C19H21NO3/c1-2-8-20-9-7-19-12-4-6-15(22)18(19)23-17-14(21)5-3-11(16(17)19)10-13(12)20/h2-6,12-13,15,18,21-22H,1,7-10H2/t12-,13+,15-,18-,19-/m0/s1
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
UIQMVEYFGZJHCZ-SSTWWWIQSA-N
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
C=CCN1CC[C@]23[C@@H]4[C@H]1CC5=C2C(=C(C=C5)O)O[C@H]3[C@H](C=C4)O
Computed by OEChem 2.3.0 (PubChem release 2024.12.12)
C19H21NO3
Computed by PubChem 2.2 (PubChem release 2021.10.14)
- Allylnormorphine
- Hydrobromide, Nalorphine
- Hydrochloride, Nalorphine
- Lethidrone
- Nalorphine
- Nalorphine Hydrobromide
- Nalorphine Hydrochloride
- Nalorphine, (14 alpha)-Isomer
- Nalorphine, L-tartrate (1:1)
- Nalorphine
- Allorphine
- Anthorphine
- Antorphine
- Nalorphinium
- Antorfin
- Antorphin
- Nalorphin
- Nalorfina
- Anarcon
- Nallin
- Norfin
- 62-67-9
- Acetorfin [Czech]
- Nalline
- Nalorfina [DCIT]
- Nalorphinum
- Acetorfin
- Normorphine, N-allyl-
- Nalorphine [INN:BAN]
- Nalorphinum [INN-Latin]
- N-Allylnormorphine
- N-Allyl-N-desmethylmorphine
- Nalorphine serb
- HSDB 3278
- UNII-U59WB2WRY2
- EINECS 200-546-1
- U59WB2WRY2
- N-allyl-normorphine
- Nalorphine (INN)
- Allylnormorphine
- NALORPHINE [MI]
- NALORPHINE [INN]
- NALORPHINE [HSDB]
- NALORPHINE [MART.]
- NALORPHINE [WHO-DD]
- Morphinan-3,6-diol, 7,8-didehydro-4,5-epoxy-17-(2-propenyl)-, (5alpha,6alpha)-
- N-Allyl-7,8-dehydro-4,5-epoxy-3,6-dihydroxymorphinan
- CHEBI:7458
- DTXSID3023348
- 7,8-Didehydro-4,5-epoxy-17-(2-propenyl)morphinan-3,6-diol
- Morphinan-3,6-alpha-diol, 17-allyl-7,8-didehydro-4,5-alpha-epoxy-
- DEA No. 9400
- Nalorphinum (INN-Latin)
- NALORPHINE (MART.)
- Letidron
- DEA Code 9400
- MORPHINAN-3,6-DIOL, 7,8-DIDEHYDRO-4,5-EPOXY-17-(2-PROPENYL)-(5.ALPHA.,6.ALPHA.)-
- (1S,5R,13R,14S,17R)-4-(prop-2-en-1-yl)-12-oxa-4-azapentacyclo[9.6.1.0^{1,13}.0^{5,17}.0^{7,18}]octadeca-7(18),8,10,15-tetraene-10,14-diol
- (4R,4aR,7S,7aR,12bS)-3-allyl-2,4,4a,7,7a,13-hexahydro-1H-4,12-methanobenzofuro[3,2-e]isoquinoline-7,9-diol
- (4R,4aR,7S,7aR,12bS)-3-prop-2-enyl-2,4,4a,7,7a,13-hexahydro-1H-4,12-methanobenzofuro[3,2-e]isoquinoline-7,9-diol
- NCGC00168255-01
- (-)-Nalorphine
- (1S,5R,13R,14S,17R)-4-(prop-2-en-1-yl)-12-oxa-4-azapentacyclo(9.6.1.0^(1,13).0^(5,17).0^(7,18))octadeca-7(18),8,10,15-tetraene-10,14-diol
- (4R,4aR,7S,7aR,12bS)-3-allyl-2,4,4a,7,7a,13-hexahydro-1H-4,12-methanobenzofuro(3,2-e)isoquinoline-7,9-diol
- (4R,4aR,7S,7aR,12bS)-3-prop-2-enyl-2,4,4a,7,7a,13-hexahydro-1H-4,12-methanobenzofuro(3,2-e)isoquinoline-7,9-diol
- Nalorphine serb (TN)
- SCHEMBL38920
- 17-Allyl-7,8-didehydro-4,5.alpha.-epoxymorphinan-3,6.alpha.-diol
- CHEMBL415284
- DTXCID303348
- GTPL1629
- V03AB02
- Morphinan-3,6-diol, 7,8-didehydro-4,5-epoxy-17-(2-propen-1-yl)-, (5.alpha.,6.alpha.)-
- BDBM50367061
- PDSP2_001572
- DB11490
- NS00008614
- D08247
- Q2622916
- 17-Allyl-7,8-didehydro-4,5alpha-epoxymorphinan-3,6alpha-diol
- Morphinan-3,6alpha-diol, 17-allyl-7,8-didehydro-4,5alpha-epoxy-
- (5alpha,6alpha)-17-prop-2-en-1-yl-7,8-didehydro-4,5-epoxymorphinan-3,6-diol
- (5alpha,6alpha)-7,8-Didehydro-4,5-epoxy-17-(2-propenyl)morphinan-3,6-diol
- Morphinan-3,6-diol, 7,8-didehydro-4,5-epoxy-17-(2-propen-1-yl)-, (5alpha,6alpha)-
- MORPHINAN-3,6-DIOL, 7,8-DIDEHYDRO-4,5-EPOXY-17-(2-PROPENYL)-(5ALPHA,6ALPHA)-
Property Name
Property Value
Reference
Property Name
Molecular Weight
Property Value
311.4 g/mol
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
XLogP3
Property Value
1.9
Reference
Computed by XLogP3 3.0 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Donor Count
Property Value
2
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Acceptor Count
Property Value
4
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Rotatable Bond Count
Property Value
2
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Exact Mass
Property Value
311.15214353 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Monoisotopic Mass
Property Value
311.15214353 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Topological Polar Surface Area
Property Value
52.9 Ų
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Heavy Atom Count
Property Value
23
Reference
Computed by PubChem
Property Name
Formal Charge
Property Value
0
Reference
Computed by PubChem
Property Name
Complexity
Property Value
549
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Isotope Atom Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Atom Stereocenter Count
Property Value
5
Reference
Computed by PubChem
Property Name
Undefined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Covalently-Bonded Unit Count
Property Value
1
Reference
Computed by PubChem
Property Name
Compound Is Canonicalized
Property Value
Yes
Reference
Computed by PubChem (release 2021.10.14)
Crystals (from diethyl ether)
Lide, D.R. CRC Handbook of Chemistry and Physics 88TH Edition 2007-2008. CRC Press, Taylor & Francis, Boca Raton, FL 2007, p. 3-382
208 °C
Lide, D.R. CRC Handbook of Chemistry and Physics 88TH Edition 2007-2008. CRC Press, Taylor & Francis, Boca Raton, FL 2007, p. 3-382
MP: 260-263 °C; max absorption (water): 285 nm; min: 260 nm; pH of 0.5% aqueous solution: 5.0; crystals from alcohol; moderately soluble in alcohol; soluble in water /Nalorphine hydrochloride/
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. Whitehouse Station, NJ: Merck and Co., Inc., 2006., p. 1101
ODORLESS; WHITE OR PRACTICALLY WHITE, CRYSTALLINE POWDER; INSOL IN CHLOROFORM & ETHER; SOL IN DIL ALKALI HYDROXIDE /NALORPHINE HYDROCHLORIDE/
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 1042
Lide, D.R. CRC Handbook of Chemistry and Physics 88TH Edition 2007-2008. CRC Press, Taylor & Francis, Boca Raton, FL 2007, p. 3-382
Sparingly soluble in ether; soluble in chloroform, dilute alkalies
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. Whitehouse Station, NJ: Merck and Co., Inc., 2006., p. 1101
log Kow = 1.86
Hansch, C., Leo, A., D. Hoekman. Exploring QSAR - Hydrophobic, Electronic, and Steric Constants. Washington, DC: American Chemical Society., 1995., p. 162
Specific optical rotation: -155.3 °C (c = 3 in methanol)
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. Whitehouse Station, NJ: Merck and Co., Inc., 2006., p. 1101
When heated to decomposition it emits very toxic fumes of /nitrogen oxides/.
Lewis, R.J. Sr. (ed) Sax's Dangerous Properties of Industrial Materials. 11th Edition. Wiley-Interscience, Wiley & Sons, Inc. Hoboken, NJ. 2004., p. 108
pKa = 7.64
Sangster J; LOGKOW Databank. Sangster Res. Lab., Montreal Quebec, Canada (1994)
Decomp at 258-259 °C; crystals from alc; soluble in water /Nalorphine hydrobromide/
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. Whitehouse Station, NJ: Merck and Co., Inc., 2006., p. 1101
Pharmaceuticals -> Listed in ZINC15
S55 | ZINC15PHARMA | Pharmaceuticals from ZINC15 | DOI:10.5281/zenodo.3247749
Pharmaceuticals -> Synthetic Cannabinoids or Psychoactive Compounds
S58 | PSYCHOCANNAB | Synthetic Cannabinoids and Psychoactive Compounds | DOI:10.5281/zenodo.3247723
Pharmaceuticals -> Steroids
S56 | UOATARGPHARMA | Target Pharmaceutical/Drug List from University of Athens | DOI:10.5281/zenodo.3248837
Pharmaceuticals
S72 | NTUPHTW | Pharmaceutically Active Substances from National Taiwan University | DOI:10.5281/zenodo.3955664
MoNA ID
MS Category
Experimental
MS Type
LC-MS
MS Level
MS1
Instrument
ZQ, Waters
Instrument Type
LC-ESI-Q
Ionization
ESI
Ionization Mode
positive
Retention Time
7.000 min
Top 5 Peaks
312 100
201 47.45
181 27.83
185 23.92
209 21.92
License
CC BY-NC
MoNA ID
MS Category
Experimental
MS Type
LC-MS
MS Level
MS1
Instrument
ZQ, Waters
Instrument Type
LC-ESI-Q
Ionization
ESI
Ionization Mode
positive
Retention Time
7.000 min
Top 5 Peaks
312 100
313 17.22
201 16.82
270 9.81
229 8.21
License
CC BY-NC
UV max: 285 nm (in acid); 298 nm (in alkali)
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. Whitehouse Station, NJ: Merck and Co., Inc., 2006., p. 1101
Follow these links to do a live 2D search or do a live 3D search for this compound, sorted by annotation score. This section is deprecated (see here for details), but these live search links provide equivalent functionality to the table that was previously shown here.
Same Connectivity Count
Same Stereo Count
Same Isotope Count
Same Parent, Connectivity Count
Same Parent, Stereo Count
Same Parent, Isotope Count
Same Parent, Exact Count
Mixtures, Components, and Neutralized Forms Count
Similar Compounds (2D)
Similar Conformers (3D)
Used to reverse opioid overdose.
Narcotic Antagonists
National Library of Medicine's Medical Subject Headings online file (MeSH, 2009)
Nalorphine is a narcotic antagonist that is used to reverse respiratory depression from narcotic overdose. It has been used either alone or in combination with meperidine or morphine during labor to reduce neonatal /respiratory/ depression. Nalorphine has also been given to the newborn to prevent neonatal asphyxia. /Former/
Briggs, G.G, R.K. Freeman, S.J. Yaffe. A Reference Guide to Fetal and Neonatal Risk. Drugs in Pregnancy and Lactation. 4th ed. Baltimore, MD: Williams & Wilkins 1994., p. 610
Nalorphine is a narcotic antagonist with some agonist properties that reduces or abolishes the depressant actions of morphine and other narcotic substances but not those of barbiturates or other non-narcotic depressants. Nalorphine has analgesic properties but is unsuitable for use as analgesic because of its unpleasant side-effects. /Former/
Reynolds, J.E.F., Prasad, A.B. (eds.) Martindale-The Extra Pharmacopoeia. 28th ed. London: The Pharmaceutical Press, 1982., p. 1032
MEDICATION (Vet): as pharmacological antagonist to resp depression, hypotension, depression, etc, produced by narcotics such as morphine, fentanyl, codeine, meperidine, methadone, hydromorphone, thiambutene, cyprenorphine, etorphene, etc, but not that produced by many anesthetics such as barbiturates, ether, chloral hydrate, etc. Despite fact that it is strong antagonist to most narcotic drug effects, it is synergistic with their antitussive effects, and is also antitussive by itself ... /SRP: former use/
Rossoff, I.S. Handbook of Veterinary Drugs. New York: Springer Publishing Company, 1974., p. 375
For more Therapeutic Uses (Complete) data for NALORPHINE (7 total), please visit the HSDB record page.
In patients given narcotic antagonists who have apparently recovered from their overdose, observation must be continued for up to 48 hours as the half-life of the antagonist was generally shorter than that of the narcotic.
Reynolds, J.E.F., Prasad, A.B. (eds.) Martindale-The Extra Pharmacopoeia. 28th ed. London: The Pharmaceutical Press, 1982., p. 1032
Some mixed agonist-antagonist drugs such as ... nalorphine, can produce severe psychotomimetic effects that are not reversible with naloxone (suggesting that these undesirable side effects are not mediated through classical opioid receptors). Also, ... nalorphine can precipatate withdrawal in opioid-tolerant patients. For these reasons, the clinical use of these mixed agonist-antagonist drugs is limited.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 11th ed. New York, NY: McGraw-Hill, 2006., p. 556
FDA Pregnancy Risk Category: D /POSITIVE EVIDENCE OF RISK. Studies in humans, or investigational or post-marketing data, have demonstrated fetal risk. Nevertheless, potential benefits from the use of the drug may outweigh the potential risk. For example, the drug may be acceptable if needed in a life-threatening situation or serious disease for which safer drugs cannot be used or are ineffective./ /Former/
Briggs, G.G, R.K. Freeman, S.J. Yaffe. A Reference Guide to Fetal and Neonatal Risk. Drugs in Pregnancy and Lactation. 4th ed. Baltimore, MD: Williams & Wilkins 1994., p. 610
... After chronic admin of high dosage, abrupt discontinuation ... causes characteristic withdrawal syndrome ... One early sign is repeated brief episodes of sensation that is described by some subjects as "electric shocks to head" and by others as light-headedness or fainting spells ... not ... convulsive phenomena ...
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 275
For more Drug Warnings (Complete) data for NALORPHINE (7 total), please visit the HSDB record page.
There was no maximum dose of nalorphine; each patient should be given the dose required to cope with his condition. Doses of up to 105 mg have been given in 1 hour without respiratory depression.
Reynolds, J.E.F., Prasad, A.B. (eds.) Martindale-The Extra Pharmacopoeia. 28th ed. London: The Pharmaceutical Press, 1982., p. 1032
Healthy subjects given progressively larger doses of nalorphine became tolerant to its effects and were cross tolerant to the effects of cyclazocine. On withdrawal, an abstinence syndrome developed which was milder than that for morphine but similar to cyclazocine.
Reynolds, J.E.F., Prasad, A.B. (eds.) Martindale-The Extra Pharmacopoeia. 28th ed. London: The Pharmaceutical Press, 1982., p. 1031
Narcotic Antagonists
Agents inhibiting the effect of narcotics on the central nervous system. (See all compounds classified as Narcotic Antagonists.)
Nalorphine is poorly absorbed when given by mouth. When administered by injection, it readily passes into the brain and across the placenta. It is largely metabolised in the liver and excreted in the urine. About 2 to 6% of the dose is excreted unchanged in the urine.
Reynolds, J.E.F., Prasad, A.B. (eds.) Martindale-The Extra Pharmacopoeia. 28th ed. London: The Pharmaceutical Press, 1982., p. 1032
... The brain/plasma ratios and degree of plasma-protein binding were significantly higher for naloxone as compared to nalorphine. The amounts of free naloxone excreted as a percentage of the dose in urine and feces 96 hours after injection of the 10 mg/kg sc dose were 4.1 and 3.9 (for nalorphine 4.7 and 8.3); conjugated drug 15.4 and 1.2 (for nalorphine 13 and 0.9); total radioactivity 43.3 and 20.9 (for nalorphine 34.8 and 19.2), respectively. ...
Misra AL et al; J Pharmacol Exp Ther 196 (2): 257-68 (1976).
In this study, the elimination of nalorphine was investigated to characterize the relation between renal and hepatic excretion of organic cations. Nalorphine is excreted effectively both via kidney and liver. However, its hepatic excretion dominates in adult rats. In young, 20-day-old animals biliary nalorphine elimination is immature and the excreted amounts are significantly lower. Renal excretion of nalorphine is quite similar in rats of both ages. After bile duct ligation renal excretion of nalorphine increases significantly in adult rats whereas it remains unchanged in young ones. Remarkably, after bilateral nephrectomy hepatic elimination of nalorphine is even diminished in both age groups. In further experiments renal excretion of nalorphine could be stimulated in adult rats after repeated administration of trometamol, triiodothyronine, or dexamethasone; these treatments had no consequences on biliary secretion of nalorphine.
Fleck C et al; Exp Pathol. 1988;34(3):171-80 (1988).
Following parenteral admin of nalorphine, concn in brain are 3-4 times higher than after comparable doses of morphine. Brain concn fall rapidly and only trace amt are found after 4 hr.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 275
First-pass effect of nalorphine-HCl, about 50% of admin radioactivity was excreted in expired air (14)CO2 and urine (conjugated and unchanged nalorphine) by 48 hr after either iv or oral admin suggesting that absorption was essentially complete. /Nalorphine HCl/
Iwamoto K, Klaassen CD; J Pharmacol Exp Ther 203 (2): 365-76 (1977)
Nalorphine-3-glucuronide dihydrate and nalorphine-6-glucuronide were isolated as urinary metabolites of nalorphine in dogs, and nalorphine-3-ethereal sulfate and nalorphine-3-glucuronide dihydrate were isolated in cats. ...
Yeh SY, Woods LA; Journal of Pharmaceutical Sciences 60 (1): 148-150 (1970)
Nalorphine yields 2-hydroxynalorphine & nalorphine-3-beta-d-glucuronide in rabbit; yields normorphine in rat. /from table/
Goodwin, B.L. Handbook of Intermediary Metabolism of Aromatic Compounds. New York: Wiley, 1976., p. N-1
... Nalrophine is much more effective after parenteral than oral admin, probably because of rapid biotransformation in liver (mainly through conjugation with glucuronic acid). /SRP: former use/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 275
First-pass effect of nalorphine-hydrogen chloride was measured in rats, major metabolic pathway for nalorphine was n-deallylation and glucuronidation. /Nalorphine HCl/
Iwamoto K, Klaassen CD; J Pharmacol Exp Ther 203 (2): 365-76 (1977)
The T1/2 of naloxone and nalorphine in rat brain and plasma with 1 and 10 mg/kg sc doses was 0.4 hour.
Misra AL et al; J Pharmacol Exp Ther 196 (2): 257-68 (1976).
The Kappa-3 opioid receptor subtype is found throughout the brain and participates in supraspinal analgesia. This receptor is primarily responsible for the action of nalorphine, an agonist-antagonist opioid. ... All opioid receptor subtypes are members of a family of a superfamily of membrane bound receptors that are coupled to G proteins. The G proteins are responsible for signaling the cell that the receptor has been activated and for initiating the the desired cellular effects. /Opioids/
Goldfrank, L.R., Goldfrank's Toxicologic Emergencies 8th Ed. 2006., McGraw-Hill, New York, N.Y., p. 596
Agonists selective for kappa receptors produce analgesia that has been shown in animal to be mediated primarily at spinal sites. Respiratory depressin and miosis may be less severe with kappa agonists. Instead of euphoria (/as with mu-receptors/), kappa receptor agonist produce dysphoric and psychotomimetic effects.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 11th ed. New York, NY: McGraw-Hill, 2006., p. 556
The effects of graded doses of nalorphine and morphine were studied in nondependent chronic spinal dogs. Morphine and low doses of nalorphine produced behavioral changes characterized by indifference, whereas the largest dose of nalorphine produced canine delirium indistinguishable from that produced by SKF-10, 047 or cyclazocine. Nalorphine depressed the flexor reflex; however, a plateau was observed. The data suggest that nalorphine is a partial agonist of the kappa type and a sigma agonist in addition to being a competitive antagonist at the mu receptor, and further, that the dysphoric and hallucinogenic effects of nalorphine-like drugs are due to their sigma activity.
Gilbert PE, Martin WR; Drug Alcohol Depend. 1976 Oct;1(6):373-6 (1976).
Intracellular microelectrode studies were conducted to investigate the actions of the partial agonist-antagonist nalorphine at an opiate receptor on functional frog skeletal muscle fiber membranes. In high bath concentrations (greater than or equal to 10(-4) M), nalorphine alone produces agonist actions similar to the "full" opiate agonists. These actions were (i) to depress both the sodium and potassium (gNa and gK) conductance increases due to electrical stimulation by a nonspecific local anestheticlike mechanism and (ii) to depress gNa by a specific opiate receptor mediated mechanism. In a much lower bath concentration (1 X 10(-8) M) nalorphine acts to antagonize the specific opiate receptor mediated depression of gNa produced by the "full" agonist meperidine. Thus in this preparation nalorphine, "the partial antagonist," has the same actions as naloxone, which is often considered to be a full antagonist. ...
Ary TE, Frank GB; Can J Physiol Pharmacol. 1983 Nov;61(11):1361-7 (1983).
For more Mechanism of Action (Complete) data for NALORPHINE (6 total), please visit the HSDB record page.
Biochemical research tool for studying the mechanism of narcotic action; also as an antidote for acute morphine poisoning.
Lewis, R.J. Sr.; Hawley's Condensed Chemical Dictionary 15th Edition. John Wiley & Sons, Inc. New York, NY 2007., p. 870
Therapeutic Category: Narcotic antagonist.
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. Whitehouse Station, NJ: Merck and Co., Inc., 2006., p. 1101
Therapeutic Category (Vet): Narcotic antagonist.
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. Whitehouse Station, NJ: Merck and Co., Inc., 2006., p. 1101
Medication
Medication (Vet)
Pharmaceuticals
S72 | NTUPHTW | Pharmaceutically Active Substances from National Taiwan University | DOI:10.5281/zenodo.3955664
Diacetylmorphine (heroin) is demethylated with cyanogen bromide and hydrolyzed to normorphine, which is alkylated with allyl bromide to give nalorphine.
Friderichs E et al; Ullmann's Encyclopedia of Industrial Chemistry 7th ed. (2008). NY, NY: John Wiley & Sons; Analgesics and Antipyretics. Online Posting Date: July 15, 2007
Prepd from normorphine.
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. Whitehouse Station, NJ: Merck and Co., Inc., 2006., p. 1101
Allorphine
U.S. Department of Health, Education & Welfare, Public Health Service. Center for Disease Control, National Institute for Occupational Safety & Health. Registry of Toxic Effects of Chemical Substances. 1978 edition. Washington, DC: U.S. Government Printing Office, 1979., p. 781
Anarcon
U.S. Department of Health, Education & Welfare, Public Health Service. Center for Disease Control, National Institute for Occupational Safety & Health. Registry of Toxic Effects of Chemical Substances. 1978 edition. Washington, DC: U.S. Government Printing Office, 1979., p. 781
Anthorphine
Antofin
U.S. Department of Health, Education & Welfare, Public Health Service. Center for Disease Control, National Institute for Occupational Safety & Health. Registry of Toxic Effects of Chemical Substances. 1978 edition. Washington, DC: U.S. Government Printing Office, 1979., p. 781
For more Formulations/Preparations (Complete) data for NALORPHINE (19 total), please visit the HSDB record page.
Nalorphine is listed in schedule iii of controlled substances act.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 1042
Lewis, R.J. Sr.; Hawley's Condensed Chemical Dictionary 15th Edition. John Wiley & Sons, Inc. New York, NY 2007., p. 870
Nalorphine ... no longer available in the United States.
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 517
Analyte: nalorphine hydrochloride; matrix: chemical identification; procedure: infrared absorption spectrophotometry with comparison to standards /Nalorphine hydrochloride /
U.S. Pharmacopeia. The United States Pharmacopeia, USP 31/The National Formulary, NF 26; Rockville, MD: U.S. Pharmacopeial Convention, Inc., p. 2752 (2008)
Analyte: nalorphine hydrochloride; matrix: chemical identification; procedure: ultraviolet absorption spectrophotometry with comparison to standards /Nalorphine hydrochloride /
U.S. Pharmacopeia. The United States Pharmacopeia, USP 31/The National Formulary, NF 26; Rockville, MD: U.S. Pharmacopeial Convention, Inc., p. 2752 (2008)
Analyte: nalorphine hydrochloride; matrix: chemical purity; procedure: ultraviolet absorption spectrophotometry at 285 nm with comparison to standards /Nalorphine hydrochloride /
U.S. Pharmacopeia. The United States Pharmacopeia, USP 31/The National Formulary, NF 26; Rockville, MD: U.S. Pharmacopeial Convention, Inc., p. 2752 (2008)
AMPEROMETRIC HIGH PERFORMANCE LIQ CHROMATOGRAPHIC METHOD FOR DETECTION OF NALORPHINE.
PETERSON RG ET AL; J CHROMATOGR 188 (2): 420-5 (1980)
Chemiluminescence detection was used in combination with flow injection analysis to determine > or = 1 fmol of morphine by its reaction with MnO4- in an acidic tetraphosphate soln. Structurally similar narcotics can also be determined by the same procedure. A mechanism for the chemiluminescent reaction is suggested.
Abbott RW et al; Analyst (London) 111 (6): 635-40 (1986)
Analyte: nalorphine hydrochloride; matrix: pharmaceutical preparation (injection solution); procedure: thin-layer chromatography with comparison to standards (chemical identification) /Nalorphine hydrochloride /
U.S. Pharmacopeia. The United States Pharmacopeia, USP 31/The National Formulary, NF 26; Rockville, MD: U.S. Pharmacopeial Convention, Inc., p. 2752 (2008)
Analyte: nalorphine hydrochloride; matrix: pharmaceutical preparation (injection solution); procedure: ultraviolet detection spectrophotometry at 285 nm with comparison to standards (chemical purity) /Nalorphine hydrochloride /
U.S. Pharmacopeia. The United States Pharmacopeia, USP 31/The National Formulary, NF 26; Rockville, MD: U.S. Pharmacopeial Convention, Inc., p. 2752 (2008)
Analyte: nalorphine; matrix: pharmaceutical preparation (solution); procedure: high-performance liquid chromatography with ultraviolet detection at 214 nm; limit of detection: 3.1 ng/mL
Ho S-T et al; J Chromatogr 570: 339-50 (1991). As cited in Lunn G; HPLC and CE methods for Pharmaceutical Analysis. CD-ROM. New York, NY: John Wiley & Sons (2000)
Analyte: nalorphine; matrix: urine; procedure: high-performance liquid chromatography with electrochemical detection and ultraviolet detection at 210 nm; limit of detection 40 ng/mL
Gerostamoulos J et al; J Chromatogr 617: 152-6 (1993). As cited in Lunn G; HPLC and CE methods for Pharmaceutical Analysis. CD-ROM. New York, NY: John Wiley & Sons (2000)
For more Clinical Laboratory Methods (Complete) data for NALORPHINE (10 total), please visit the HSDB record page.
SRP: Expired or waste pharmaceuticals shall carefully take into consideration applicable DEA, EPA, and FDA regulations. It is not appropriate to dispose by flushing the pharmaceutical down the toilet or discarding to trash. If possible return the pharmaceutical to the manufacturer for proper disposal being careful to properly label and securely package the material. Alternatively, the waste pharmaceutical shall be labeled, securely packaged and transported by a state licensed medical waste contractor to dispose by burial in a licensed hazardous or toxic waste landfill or incinerator.
SRP: At the time of review, regulatory criteria for small quantity disposal are subject to significant revision, however, household quantities of waste pharmaceuticals may be managed as follows: Mix with wet cat litter or coffee grounds, double bag in plastic, discard in trash.
New Zealand EPA Inventory of Chemical Status
Morphinan-3,6-diol, 7,8-didehydro-4,5-epoxy-17-(2-propenyl)-, (5.alpha.,6.alpha.)-: Does not have an individual approval but may be used under an appropriate group standard
Nalorphine hydrochloride injection. ... Indications for use. Respiratory and circulatory depression in dogs resulting from overdosage of, or unusual sensitivity to, morphine and certain other narcotics. Not for depression due to any other cause. ... Limitations: Successive doses of the drug gradually lose their analeptic effect and eventually induce respiratory depression equal to that of opiates. Therefore, do not exceed therapeutic dosage. Do not mix drug with meperidine solutions because the buffer will cause precipitation. Federal law restricts this drug to use by or on the order of a licensed veterinarian.
21 CFR 522.1452 (USFDA); U.S. National Archives and Records Administration's Electronic Code of Federal Regulations. Available from, as of October10, 2009: https://www.ecfr.gov
The Generic Animal Drug and Patent Restoration act requires that each sponsor of an approved animal drug must submit to the FDA certain information regarding patents held for the animal drug or its method of use. The Act requires that this information, as well as a list of all animal drug products approved for safety and effectiveness, be made available to the public. Nalorphine hydrochloride is included on this list. /Nalorphine hydrochloride/
US FDA/Center for Veterinary Medicine; The Green Book - On Line, Active Ingredients. Nalorphine Hydrochloride (57-29-4). Available from, as of October 11, 2009: https://www.fda.gov/AnimalVeterinary/Products/ApprovedAnimalDrugProducts/default.htm
Schedule III shall consist of the drugs and other substances, by whatever official name, common or usual name, chemical name, or brand name designated, listed in this section. Each drug or substance has been assigned the DEA Controlled Substances Code Number set forth opposite it. Nalorphine (DEA Code Number: 9400) is included on this list.
21 CFR 1308.13(d) (USDEA); U.S. National Archives and Records Administration's Electronic Code of Federal Regulations. Available from, as of October10, 2009: https://www.ecfr.gov
When both morphine and nalorphine were given together, intramuscularly, in varying dosages, the resulting effects on the respiration were complex: with 5 mg of morphine, increasing the dose of nalorphine produced greater depression than increasing it with 10 mg morphine. The interaction of the two drugs was regarded as an example of "competitive dualism". The antagonism by nalorphine of the respiratory depression produced by morphine was primarily related to differences in the intrinsic action of each drug, and not simply due to mutually competitive affinities for certain receptor sites.
Reynolds, J.E.F., Prasad, A.B. (eds.) Martindale-The Extra Pharmacopoeia. 28th ed. London: The Pharmaceutical Press, 1982., p. 1032
... Resp depressant actions of nalorphine-type antagonists may add to existing resp depression produced by CNS depressants.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 274
Nalorphine HBR reportedly enhanced depression caused by combination injection of meperidine-levallorphan in pt receiving phenelzine /and other monoamine oxidase inhibitors/ ... /Nalorphine hydrobromide/
Evaluations of Drug Interactions. 2nd ed. and supplements. Washington, DC: American Pharmaceutical Assn., 1976, 1978., p. 142
/SRP:/ Immediate first aid: Ensure that adequate decontamination has been carried out. If patient is not breathing, start artificial respiration, preferably with a demand valve resuscitator, bag-valve-mask device, or pocket mask, as trained. Perform CPR if necessary. Immediately flush contaminated eyes with gently flowing water. Do not induce vomiting. If vomiting occurs, lean patient forward or place on the left side (head-down position, if possible) to maintain an open airway and prevent aspiration. Keep patient quiet and maintain normal body temperature. Obtain medical attention. /Poisons A and B/
Currance, P.L. Clements, B., Bronstein, A.C. (Eds).; Emergency Care For Hazardous Materials Exposure. 3Rd edition, Elsevier Mosby, St. Louis, MO 2005, p. 160
/SRP:/ Basic treatment: Establish a patent airway (oropharyngeal or nasopharyngeal airway, if needed). Suction if necessary. Watch for signs of respiratory insufficiency and assist ventilations if needed. Administer oxygen by nonrebreather mask at 10 to 15 L/min. Monitor for pulmonary edema and treat if necessary ... . Monitor for shock and treat if necessary ... . Anticipate seizures and treat if necessary ... . For eye contamination, flush eyes immediately with water. Irrigate each eye continuously with 0.9% saline (NS) during transport ... . Do not use emetics. For ingestion, rinse mouth and administer 5 mL/kg up to 200 mL of water for dilution if the patient can swallow, has a strong gag reflex, and does not drool ... . Cover skin burns with dry sterile dressings after decontamination ... . /Poisons A and B/
Currance, P.L. Clements, B., Bronstein, A.C. (Eds).; Emergency Care For Hazardous Materials Exposure. 3Rd edition, Elsevier Mosby, St. Louis, MO 2005, p. 160
/SRP:/ Advanced treatment: Consider orotracheal or nasotracheal intubation for airway control in the patient who is unconscious, has severe pulmonary edema, or is in severe respiratory distress. Positive-pressure ventilation techniques with a bag valve mask device may be beneficial. Consider drug therapy for pulmonary edema ... . Consider administering a beta agonist such as albuterol for severe bronchospasm ... . Monitor cardiac rhythm and treat arrhythmias as necessary ... . Start IV administration of D5W /SRP: "To keep open", minimal flow rate/. Use 0.9% saline (NS) or lactated Ringer's if signs of hypovolemia are present. For hypotension with signs of hypovolemia, administer fluid cautiously. Watch for signs of fluid overload ... . Treat seizures with diazepam or lorazepam ... . Use proparacaine hydrochloride to assist eye irrigation ... . /Poisons A and B/
Currance, P.L. Clements, B., Bronstein, A.C. (Eds).; Emergency Care For Hazardous Materials Exposure. 3Rd edition, Elsevier Mosby, St. Louis, MO 2005, p. 160-1
/HUMAN EXPOSURE STUDIES/ The effects of nalorphine 5 mg im, a partial opiate antagonist, on circulating levels of PRL, GH, TSH, LH, FSH and cortisol were studied in six healthy men. Nalorphine produced a produced a prompt and sharp increase in serum PRL and a small, delayed rise in serum GH. Serum LH and cortisol decreased after drug administration and no change in serum FSH and TSH was observed.
Rolandi E et al; Eur J Clin Pharmacol 21 (1): 23-5 (1981).
/HUMAN EXPOSURE STUDIES/ The comparative study of 2 narcotic antagonists, naloxone and nalorphine, was performed in healthy volunteers. The influence of these drugs on the respiratory and cicularoty systems and on the psychical state was compared. The study was carried out in a double-blind, cross-over manner. Increasing doses of naloxone and placebo or nalorphine and placebo, were administered intravenously. Naloxone, even in very high doses, caused no changes in cardivascular system, acid-base balance, sensitivity of respiratory centre to carbon dioxide and psychical state of volunteers. After the administration of nalorphine, even in very small doses, changes in psychical state in all examined subjects were observed. Nalorphine caused /a/ significant change in the ventilatory response to CO2. ...
Aronski A et al: Anaesth Resusc Intensive Ther 3 (3): 221-30 (1975).
/HUMAN EXPOSURE STUDIES/ In the control trials on near-term pregnant women before tyhe onset of labor, nalorphine was found to cause a mild respiratory acidosis in the mother and metabolic acidosis in the fetus. ...
Chang A et al; Med J Aust 1 (9): 263-4 (1976).
/SIGNS AND SYMPTOMS/ Significant percentage experience unpleasant reactions that range from anxiety, "crazy feelings," and vivid, disturbing, "unreal" daydreams to frank hallucinations.
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 515
For more Human Toxicity Excerpts (Complete) data for NALORPHINE (8 total), please visit the HSDB record page.
LD50 Rat sc 474 mg/kg
Lewis, R.J. Sr. (ed) Sax's Dangerous Properties of Industrial Materials. 11th Edition. Wiley-Interscience, Wiley & Sons, Inc. Hoboken, NJ. 2004., p. 108
LD50 Rat iv 226 mg/kg
Lewis, R.J. Sr. (ed) Sax's Dangerous Properties of Industrial Materials. 11th Edition. Wiley-Interscience, Wiley & Sons, Inc. Hoboken, NJ. 2004., p. 108
LD50 Mouse oral 1140 mg/kg
Lewis, R.J. Sr. (ed) Sax's Dangerous Properties of Industrial Materials. 11th Edition. Wiley-Interscience, Wiley & Sons, Inc. Hoboken, NJ. 2004., p. 108
LD50 Mouse ip 492 mg/kg
Lewis, R.J. Sr. (ed) Sax's Dangerous Properties of Industrial Materials. 11th Edition. Wiley-Interscience, Wiley & Sons, Inc. Hoboken, NJ. 2004., p. 108
For more Non-Human Toxicity Values (Complete) data for NALORPHINE (7 total), please visit the HSDB record page.
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=UIQMVEYFGZJHCZ-SSTWWWIQSA-N
The LOTUS Initiative for Open Natural Products Research: frozen dataset union wikidata (with metadata) | DOI:10.5281/zenodo.5794106
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/(-)-Nalorphinehttps://commonchemistry.cas.org/detail?cas_rn=62-67-9
- ChemIDplusNalorphine [INN:BAN]https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000062679ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- DrugBankLICENSECreative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode)https://www.drugbank.ca/legal/terms_of_useNalorphinehttps://www.drugbank.ca/drugs/DB11490
- EPA DSSToxCompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-notice
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- Hazardous Substances Data Bank (HSDB)
- New Zealand Environmental Protection Authority (EPA)LICENSEThis work is licensed under the Creative Commons Attribution-ShareAlike 4.0 International licence.https://www.epa.govt.nz/about-this-site/general-copyright-statement/Morphinan-3,6-diol, 7,8-didehydro-4,5-epoxy-17-(2-propenyl)-, (5.alpha.,6.alpha.)-https://www.epa.govt.nz/industry-areas/hazardous-substances/guidance-for-importers-and-manufacturers/hazardous-substances-databases/
- ChEBI
- LOTUS - the natural products occurrence databaseLICENSEThe code for LOTUS is released under the GNU General Public License v3.0.https://lotus.nprod.net/Nalorphinehttps://www.wikidata.org/wiki/Q2622916LOTUS Treehttps://lotus.naturalproducts.net/
- Open TargetsLICENSEDatasets generated by the Open Targets Platform are freely available for download.https://platform-docs.opentargets.org/licence
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.htmlChEMBL Protein Target Treehttps://www.ebi.ac.uk/chembl/g/#browse/targets
- Comparative Toxicogenomics Database (CTD)LICENSEIt is to be used only for research and educational purposes. Any reproduction or use for commercial purpose is prohibited without the prior express written permission of NC State University.http://ctdbase.org/about/legal.jsp
- IUPHAR/BPS Guide to PHARMACOLOGYLICENSEThe Guide to PHARMACOLOGY database is licensed under the Open Data Commons Open Database License (ODbL) https://opendatacommons.org/licenses/odbl/. Its contents are licensed under a Creative Commons Attribution-ShareAlike 4.0 International License (http://creativecommons.org/licenses/by-sa/4.0/)https://www.guidetopharmacology.org/about.jsp#licenseGuide to Pharmacology Target Classificationhttps://www.guidetopharmacology.org/targets.jsp
- Therapeutic Target Database (TTD)
- Japan Chemical Substance Dictionary (Nikkaji)
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.htmlAnatomical Therapeutic Chemical (ATC) classificationhttp://www.genome.jp/kegg-bin/get_htext?br08303.kegTarget-based classification of drugshttp://www.genome.jp/kegg-bin/get_htext?br08310.keg
- Natural Product Activity and Species Source (NPASS)
- MassBank of North America (MoNA)LICENSEThe content of the MoNA database is licensed under CC BY 4.0.https://mona.fiehnlab.ucdavis.edu/documentation/license
- Metabolomics Workbench
- NCI Thesaurus (NCIt)LICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuseNCI Thesaurushttps://ncit.nci.nih.gov
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/NalorphineNORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- PharosLICENSEData accessed from Pharos and TCRD is publicly available from the primary sources listed above. Please respect their individual licenses regarding proper use and redistribution.https://pharos.nih.gov/about
- SpectraBase
- Springer Nature
- Thieme ChemistryLICENSEThe Thieme Chemistry contribution within PubChem is provided under a CC-BY-NC-ND 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc-nd/4.0/
- WHO Anatomical Therapeutic Chemical (ATC) ClassificationLICENSEUse of all or parts of the material requires reference to the WHO Collaborating Centre for Drug Statistics Methodology. Copying and distribution for commercial purposes is not allowed. Changing or manipulating the material is not allowed.https://www.whocc.no/copyright_disclaimer/
- Wikidatanalorphinehttps://www.wikidata.org/wiki/Q2622916
- WikipediaThiocolchicosidehttps://en.wikipedia.org/wiki/ThiocolchicosideNalorphinehttps://en.wikipedia.org/wiki/Nalorphine
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlNarcotic Antagonistshttps://www.ncbi.nlm.nih.gov/mesh/68009292
- PubChem
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 403379320https://pubchem.ncbi.nlm.nih.gov/substance/403379320
- NCBI
CONTENTS