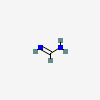Formamidine
PubChem CID
68047
Molecular Formula
Synonyms
- Formamidine
- Methanimidamide
- formimidamide
- imidoformamide
- Methanoic acid amidine
Molecular Weight
44.056 g/mol
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Dates
- Create:2005-03-26
- Modify:2025-01-11
Description
Formamidine is the smallest member of the class of carboxamidines being formic acid with the O and OH groups from the carboxy function replaced by NH and NH2 groups respectively. The parent of the class of formamidines. It has a role as an EC 1.14.13.39 (nitric oxide synthase) inhibitor. It is a carboxamidine, a member of formamidines and a one-carbon compound. It is functionally related to a formic acid.
Chemical Structure Depiction
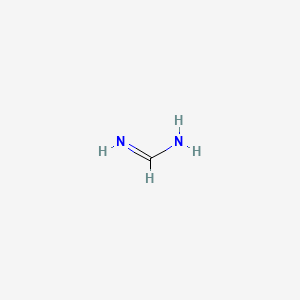
methanimidamide
Computed by Lexichem TK 2.7.0 (PubChem release 2021.10.14)
InChI=1S/CH4N2/c2-1-3/h1H,(H3,2,3)
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
PNKUSGQVOMIXLU-UHFFFAOYSA-N
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
C(=N)N
Computed by OEChem 2.3.0 (PubChem release 2024.12.12)
CH4N2
Computed by PubChem 2.2 (PubChem release 2021.10.14)
6313-33-3
463-52-5 (Parent)
- formamidine
- formamidine acetate
- formamidine hydrochloride
- formamidinium
- methanimidamide
- Formamidine
- Methanimidamide
- formimidamide
- imidoformamide
- Methanoic acid amidine
- 463-52-5
- Formamidinium
- M8KP3Q82AG
- HC(=NH)-NH2
- CHEBI:38477
- PNKUSGQVOMIXLU-UHFFFAOYSA-
- DTXSID00870543
- PFC3YH7YHK
- UNII-PFC3YH7YHK
- UNII-M8KP3Q82AG
- DTXCID20818260
- PNKUSGQVOMIXLU-UHFFFAOYSA-N
- EINECS 228-639-2
- BBL011114
- NSC 39860
- STK802376
- AKOS005622690
- 87667-19-4
- NS00040720
- Q27117873
Property Name
Property Value
Reference
Property Name
Molecular Weight
Property Value
44.056 g/mol
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
XLogP3-AA
Property Value
-0.8
Reference
Computed by XLogP3 3.0 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Donor Count
Property Value
2
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Acceptor Count
Property Value
1
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Rotatable Bond Count
Property Value
0
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Exact Mass
Property Value
44.037448136 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Monoisotopic Mass
Property Value
44.037448136 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Topological Polar Surface Area
Property Value
49.9 Ų
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Heavy Atom Count
Property Value
3
Reference
Computed by PubChem
Property Name
Formal Charge
Property Value
0
Reference
Computed by PubChem
Property Name
Complexity
Property Value
10.3
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Isotope Atom Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Covalently-Bonded Unit Count
Property Value
1
Reference
Computed by PubChem
Property Name
Compound Is Canonicalized
Property Value
Yes
Reference
Computed by PubChem (release 2021.10.14)
1D NMR Spectra
Follow these links to do a live 2D search or do a live 3D search for this compound, sorted by annotation score. This section is deprecated (see here for details), but these live search links provide equivalent functionality to the table that was previously shown here.
Same Connectivity Count
Same Parent, Connectivity Count
Same Parent, Exact Count
Mixtures, Components, and Neutralized Forms Count
Similar Compounds (2D)
Similar Conformers (3D)
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=PNKUSGQVOMIXLU-UHFFFAOYSA-N
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/
- ChemIDplusFormamidine hydrochloridehttps://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0006313333ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- EPA DSSToxMethanimidamidehttps://comptox.epa.gov/dashboard/DTXSID00870543CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- ChEBI
- Human Metabolome Database (HMDB)LICENSEHMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications.http://www.hmdb.ca/citingFormamidinehttp://www.hmdb.ca/metabolites/HMDB0252441
- Japan Chemical Substance Dictionary (Nikkaji)
- Metabolomics Workbench
- NMRShiftDB
- SpectraBase
- Springer Nature
- Thieme ChemistryLICENSEThe Thieme Chemistry contribution within PubChem is provided under a CC-BY-NC-ND 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc-nd/4.0/
- Wikidataformamidinehttps://www.wikidata.org/wiki/Q27117873
- Wiley
- PubChem
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlformamidinehttps://www.ncbi.nlm.nih.gov/mesh/67077922
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/NORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 403380814https://pubchem.ncbi.nlm.nih.gov/substance/403380814
CONTENTS