coproporphyrinogen III(4-)
PubChem CID
20849114
Molecular Formula
Synonyms
- coproporphyrinogen III(4-)
- CHEBI:57309
- Q27124412
- 3-[8,12,17-tris(2-carboxylatoethyl)-3,7,13,18-tetramethyl-5,10,15,20,21,22,23,24-octahydroporphyrin-2-yl]propanoate
Molecular Weight
656.7 g/mol
Computed by PubChem 2.1 (PubChem release 2021.05.07)
Parent Compound
Dates
- Create:2007-12-05
- Modify:2025-01-18
Description
Coproporphyrinogen III(4-) is tetracarboxylate anion of coproporphyrinogen III. It has a role as a human metabolite and a Saccharomyces cerevisiae metabolite. It is a conjugate base of a coproporphyrinogen III.
Chemical Structure Depiction
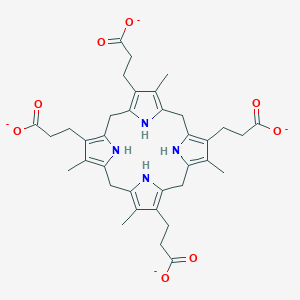
3-[8,12,17-tris(2-carboxylatoethyl)-3,7,13,18-tetramethyl-5,10,15,20,21,22,23,24-octahydroporphyrin-2-yl]propanoate
Computed by LexiChem 2.6.6 (PubChem release 2019.06.18)
InChI=1S/C36H44N4O8/c1-17-21(5-9-33(41)42)29-14-27-19(3)22(6-10-34(43)44)30(39-27)15-28-20(4)24(8-12-36(47)48)32(40-28)16-31-23(7-11-35(45)46)18(2)26(38-31)13-25(17)37-29/h37-40H,5-16H2,1-4H3,(H,41,42)(H,43,44)(H,45,46)(H,47,48)/p-4
Computed by InChI 1.0.5 (PubChem release 2019.06.18)
NIUVHXTXUXOFEB-UHFFFAOYSA-J
Computed by InChI 1.0.5 (PubChem release 2019.06.18)
CC1=C2CC3=C(C(=C(N3)CC4=C(C(=C(N4)CC5=C(C(=C(N5)CC(=C1CCC(=O)[O-])N2)C)CCC(=O)[O-])C)CCC(=O)[O-])CCC(=O)[O-])C
Computed by OEChem 2.3.0 (PubChem release 2024.12.12)
C36H40N4O8-4
Computed by PubChem 2.1 (PubChem release 2019.06.18)
Property Name
Property Value
Reference
Property Name
Molecular Weight
Property Value
656.7 g/mol
Reference
Computed by PubChem 2.1 (PubChem release 2021.05.07)
Property Name
XLogP3-AA
Property Value
6.2
Reference
Computed by XLogP3 3.0 (PubChem release 2019.06.18)
Property Name
Hydrogen Bond Donor Count
Property Value
4
Reference
Computed by Cactvs 3.4.6.11 (PubChem release 2019.06.18)
Property Name
Hydrogen Bond Acceptor Count
Property Value
8
Reference
Computed by Cactvs 3.4.6.11 (PubChem release 2019.06.18)
Property Name
Rotatable Bond Count
Property Value
8
Reference
Computed by Cactvs 3.4.6.11 (PubChem release 2019.06.18)
Property Name
Exact Mass
Property Value
656.28461425 Da
Reference
Computed by PubChem 2.1 (PubChem release 2021.05.07)
Property Name
Monoisotopic Mass
Property Value
656.28461425 Da
Reference
Computed by PubChem 2.1 (PubChem release 2021.05.07)
Property Name
Topological Polar Surface Area
Property Value
224 Ų
Reference
Computed by Cactvs 3.4.6.11 (PubChem release 2019.06.18)
Property Name
Heavy Atom Count
Property Value
48
Reference
Computed by PubChem
Property Name
Formal Charge
Property Value
-4
Reference
Computed by PubChem
Property Name
Complexity
Property Value
1150
Reference
Computed by Cactvs 3.4.6.11 (PubChem release 2019.06.18)
Property Name
Isotope Atom Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Covalently-Bonded Unit Count
Property Value
1
Reference
Computed by PubChem
Property Name
Compound Is Canonicalized
Property Value
Yes
Reference
Computed by PubChem (release 2010.01.29)
Follow these links to do a live 2D search or do a live 3D search for this compound, sorted by annotation score. This section is deprecated (see here for details), but these live search links provide equivalent functionality to the table that was previously shown here.
Same Parent, Exact Count
Mixtures, Components, and Neutralized Forms Count
Similar Compounds (2D)
Similar Conformers (3D)
Same Count
- ChEBICoproporphyrinogen III(4-)https://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:57309
- Natural Product Activity and Species Source (NPASS)
- Rhea - Annotated Reactions DatabaseLICENSERhea has chosen to apply the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0/). This means that you are free to copy, distribute, display and make commercial use of the database in all legislations, provided you credit (cite) Rhea.https://www.rhea-db.org/help/license-disclaimer
- Wikidatacoproporphyrinogen III(4-)https://www.wikidata.org/wiki/Q27124412
- PubChem
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
CONTENTS

 CID 321 (coproporphyrinogen III)
CID 321 (coproporphyrinogen III)