cis-Pinosylvin
PubChem CID
9548840
Molecular Formula
Synonyms
- cis-pinosylvin
- 3,5-Stilbenediol, (Z)-
- UNII-SUT6G9WH0N
- SUT6G9WH0N
- (z)-3,5-dihydroxystilbene
Molecular Weight
212.24 g/mol
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Dates
- Create:2006-10-04
- Modify:2025-01-04
Description
cis-Pinosylvin has been reported in Alpinia hainanensis, Pinus armandii, and Pinus morrisonicola with data available.
Chemical Structure Depiction
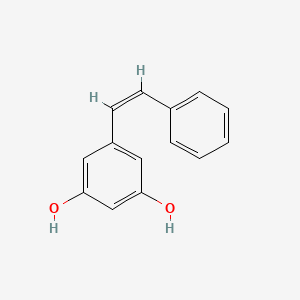
5-[(Z)-2-phenylethenyl]benzene-1,3-diol
Computed by Lexichem TK 2.7.0 (PubChem release 2021.10.14)
InChI=1S/C14H12O2/c15-13-8-12(9-14(16)10-13)7-6-11-4-2-1-3-5-11/h1-10,15-16H/b7-6-
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
YCVPRTHEGLPYPB-SREVYHEPSA-N
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
C1=CC=C(C=C1)/C=C\C2=CC(=CC(=C2)O)O
Computed by OEChem 2.3.0 (PubChem release 2024.12.12)
C14H12O2
Computed by PubChem 2.2 (PubChem release 2021.10.14)
106325-78-4
- cis-pinosylvin
- 3,5-Stilbenediol, (Z)-
- UNII-SUT6G9WH0N
- SUT6G9WH0N
- (z)-3,5-dihydroxystilbene
- CHEBI:36010
- 106325-78-4
- 5-[(Z)-2-phenylvinyl]benzene-1,3-diol
- 5-[(1Z)-2-phenylethenyl]benzene-1,3-diol
- SCHEMBL9419839
- (Z)-3,5-STILBENEDIOL
- CHEMBL2203685
- 3,5-DIHYDROXYSTILBENE, (Z)-
- Q27116666
- 1,3-BENZENEDIOL, 5-((1Z)-2-PHENYLETHENYL)-
- 1,3-BENZENEDIOL, 5-(2-PHENYLETHENYL)-, (Z)-
Property Name
Property Value
Reference
Property Name
Molecular Weight
Property Value
212.24 g/mol
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
XLogP3-AA
Property Value
3.5
Reference
Computed by XLogP3 3.0 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Donor Count
Property Value
2
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Acceptor Count
Property Value
2
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Rotatable Bond Count
Property Value
2
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Exact Mass
Property Value
212.083729621 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Monoisotopic Mass
Property Value
212.083729621 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Topological Polar Surface Area
Property Value
40.5 Ų
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Heavy Atom Count
Property Value
16
Reference
Computed by PubChem
Property Name
Formal Charge
Property Value
0
Reference
Computed by PubChem
Property Name
Complexity
Property Value
221
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Isotope Atom Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Bond Stereocenter Count
Property Value
1
Reference
Computed by PubChem
Property Name
Undefined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Covalently-Bonded Unit Count
Property Value
1
Reference
Computed by PubChem
Property Name
Compound Is Canonicalized
Property Value
Yes
Reference
Computed by PubChem (release 2021.10.14)
- 13C nuclear magnetic resonance spectrum
- Angular frequency
- Boiling point
- Chemical shift
- Chemical structure
- Clearing parameter
- Crystal structure
- Crystal-to-crystal transition
- Density
- Diamagnetic susceptibility
- Dielectric constant
- Excess enthalpy
- External quantum efficiency
- Formula unit
- Formula weight
- Fusion temperature
- Gross formula
- Heat of solution
- Heat of sublimation
- Ionic conductivity
- Liquid crystalline phase
- Luminescence
- Luminescence emission linewidth
- Magnetic susceptibility
- Melting temperature
- Melting transition
- Mixing enthalpy
- Molar conductivity
- Molecular structure
- Nuclear magnetic resonance
- Nuclear quadrupole resonance spectroscopy
- Optical coefficient
- Phase transition
- Polarization degree
- Quadrupole coupling
- Refractive index
- Solid-to-solid transition
- Space group
- Spin-spin coupling constant
- Surface tension
- Transition enthalpy
- Transition pressure
- Unit cell
- Unit cell parameter
- Vapor pressure
- Vapor-liquid equilibrium
- Viscosity
Follow these links to do a live 2D search or do a live 3D search for this compound, sorted by annotation score. This section is deprecated (see here for details), but these live search links provide equivalent functionality to the table that was previously shown here.
Same Connectivity Count
Same Parent, Connectivity Count
Similar Compounds (2D)
Similar Conformers (3D)
Same Count
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=YCVPRTHEGLPYPB-SREVYHEPSA-N
The LOTUS Initiative for Open Natural Products Research: frozen dataset union wikidata (with metadata) | DOI:10.5281/zenodo.5794106
- ChEBI
- LOTUS - the natural products occurrence databaseLICENSEThe code for LOTUS is released under the GNU General Public License v3.0.https://lotus.nprod.net/cis-Pinosylvinhttps://www.wikidata.org/wiki/Q27104325LOTUS Treehttps://lotus.naturalproducts.net/
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.html
- ChemIDplus3,5-Stilbenediol, (Z)-https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0106325784ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking3,5-STILBENEDIOL, (Z)-https://gsrs.ncats.nih.gov/ginas/app/beta/substances/SUT6G9WH0N
- FooDBLICENSEFooDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (FooDB) and the original publication.https://foodb.ca/aboutPinosylvinhttps://foodb.ca/compounds/FDB002541
- Japan Chemical Substance Dictionary (Nikkaji)
- Metabolomics Workbench
- SpectraBase3,5-DIHYDROXYSTILBENE-(Z)https://spectrabase.com/spectrum/7RitGrutuyz
- Springer Nature
- SpringerMaterials
- Wikidata(Z)-3,5-stilbenediolhttps://www.wikidata.org/wiki/Q27104325
- PubChem
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 388950108https://pubchem.ncbi.nlm.nih.gov/substance/388950108
CONTENTS