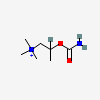
Bethanechol
- bethanechol
- 674-38-4
- Amidopropyldimethylbetaine
- Carbamyl-beta-methylcholine
- Carbamoyl-beta-methylcholine
- Create:2005-03-25
- Modify:2025-01-11
 Bethanechol Chloride (has salt form).
Bethanechol Chloride (has salt form).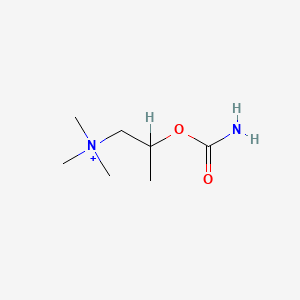
- Bethanechol
- Bethanechol Chloride
- Bethanecol
- Chloride, Bethanechol
- Duvoid
- Hermes, Myo
- Myo Hermes
- Myocholine
- Myotonachol
- Myotonine
- PMS Bethanechol Chloride
- PMS-Bethanechol Chloride
- Urecholine
- Urocarb
- bethanechol
- 674-38-4
- Amidopropyldimethylbetaine
- Carbamyl-beta-methylcholine
- Carbamoyl-beta-methylcholine
- (2-Hydroxypropyl)trimethylammonium carbamate
- 2-carbamoyloxypropyl-trimethylazanium
- 2-carbamoyloxypropyl(trimethyl)azanium
- BRN 1773706
- CHEBI:3084
- UNII-004F72P8F4
- 2-((Aminocarbonyl)oxy)-N,N,N-trimethyl-1-propanaminium
- Ammonium, (2-hydroxypropyl)trimethyl-, carbamate (ester)
- 2-carbamoyloxypropyl-trimethyl-azanium
- 004F72P8F4
- 2-(carbamoyloxy)-N,N,N-trimethylpropan-1-aminium
- 1-Propanaminium, 2-((aminocarbonyl)oxy)-N,N,N-trimethyl-
- Bethanecol
- 1-Propanaminium, 2-[(aminocarbonyl)oxy]-N,N,N-trimethyl-, (S)-
- NCGC00163217-01
- 2-carbamoyloxypropyl(trimethyl)azanium;chloride
- Carbamyl--methylcholine
- Spectrum_000084
- Prestwick0_001073
- Prestwick1_001073
- Prestwick2_001073
- Prestwick3_001073
- Spectrum2_000132
- Spectrum3_000311
- Spectrum4_000255
- Spectrum5_000892
- BETHANECHOL [VANDF]
- CHEMBL1482
- Lopac0_000304
- SCHEMBL37096
- BETHANECHOL [WHO-DD]
- BSPBio_001086
- BSPBio_001902
- GTPL297
- KBioGR_000690
- KBioSS_000504
- cid_11548
- DivK1c_000179
- SPBio_000204
- SPBio_002993
- BPBio1_001196
- DTXSID5048398
- BDBM39342
- KBio1_000179
- KBio2_000504
- KBio2_003072
- KBio2_005640
- KBio3_001402
- NINDS_000179
- HMS2089I08
- HY-B0406
- AKOS025310166
- CCG-204399
- CS-4994
- DB01019
- IDI1_000179
- NCGC00015245-03
- NCGC00015245-04
- NCGC00015245-06
- NCGC00015245-07
- NCGC00015245-08
- NCGC00015245-18
- NCGC00089770-02
- DA-71442
- SY112089
- SBI-0050292.P004
- AB00053794
- NS00007014
- 2-carbamoyloxypropyl(trimethyl)ammonium;chloride
- C06850
- AB00053794-14
- AB00053794-15
- AB00053794_16
- AB00053794_17
- 2-aminocarbonyloxypropyl(trimethyl)azanium;chloride
- Q419059
- BRD-A99833829-003-25-5
- BRD-A99833829-003-26-3
- BRD-A99833829-003-27-1
 Bethanechol Chloride (has salt form)
Bethanechol Chloride (has salt form)Use (kg; approx.) in Germany (2009): >100
Consumption (g per capita; approx.) in Germany (2009): 0.00122
Calculated removal (%): 75.1
M Chen, V Vijay, Q Shi, Z Liu, H Fang, W Tong. FDA-Approved Drug Labeling for the Study of Drug-Induced Liver Injury, Drug Discovery Today, 16(15-16):697-703, 2011. PMID:21624500 DOI:10.1016/j.drudis.2011.05.007
M Chen, A Suzuki, S Thakkar, K Yu, C Hu, W Tong. DILIrank: the largest reference drug list ranked by the risk for developing drug-induced liver injury in humans. Drug Discov Today 2016, 21(4): 648-653. PMID:26948801 DOI:10.1016/j.drudis.2016.02.015
◉ Summary of Use during Lactation
No information is available on the use of bethanechol during breastfeeding. If it is used during breastfeeding, monitor the infant for signs of cholinergic excess (diarrhea, lacrimation, and excessive salivation or urination), especially in younger, exclusively breastfed infants.
◉ Effects in Breastfed Infants
Relevant published information was not found as of the revision date.
◉ Effects on Lactation and Breastmilk
Relevant published information in nursing mothers was not found as of the revision date. In animals, cholinergic drugs increase oxytocin release, and have variable effects on serum prolactin. The prolactin level in a mother with established lactation may not affect her ability to breastfeed.
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=NZUPCNDJBJXXRF-UHFFFAOYSA-O
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/
- ChemIDplusChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- DrugBankLICENSECreative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode)https://www.drugbank.ca/legal/terms_of_useBethanecholhttps://www.drugbank.ca/drugs/DB01019
- EPA DSSToxCompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- Human Metabolome Database (HMDB)LICENSEHMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications.http://www.hmdb.ca/citingBethanecholhttp://www.hmdb.ca/metabolites/HMDB0015154
- CCSbaseCCSbase Classificationhttps://ccsbase.net/
- ChEBI
- FDA Pharm ClassesLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingFDA Pharmacological Classificationhttps://www.fda.gov/ForIndustry/DataStandards/StructuredProductLabeling/ucm162549.htm
- Open TargetsLICENSEDatasets generated by the Open Targets Platform are freely available for download.https://platform-docs.opentargets.org/licence
- Toxin and Toxin Target Database (T3DB)LICENSET3DB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (T3DB) and the original publication.http://www.t3db.ca/downloadsBethanecholhttp://www.t3db.ca/toxins/T3D2958
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.htmlChEMBL Protein Target Treehttps://www.ebi.ac.uk/chembl/g/#browse/targets
- ClinicalTrials.govLICENSEThe ClinicalTrials.gov data carry an international copyright outside the United States and its Territories or Possessions. Some ClinicalTrials.gov data may be subject to the copyright of third parties; you should consult these entities for any additional terms of use.https://clinicaltrials.gov/ct2/about-site/terms-conditions#Use
- Drug Gene Interaction database (DGIdb)LICENSEThe data used in DGIdb is all open access and where possible made available as raw data dumps in the downloads section.http://www.dgidb.org/downloadsBETHANECHOLhttps://www.dgidb.org/drugs/rxcui:19257[PHE13,TYR19]MCHhttps://www.dgidb.org/drugs/iuphar.ligand:1297SERIBANTUMABhttps://www.dgidb.org/drugs/iuphar.ligand:8297
- IUPHAR/BPS Guide to PHARMACOLOGYLICENSEThe Guide to PHARMACOLOGY database is licensed under the Open Data Commons Open Database License (ODbL) https://opendatacommons.org/licenses/odbl/. Its contents are licensed under a Creative Commons Attribution-ShareAlike 4.0 International License (http://creativecommons.org/licenses/by-sa/4.0/)https://www.guidetopharmacology.org/about.jsp#licenseGuide to Pharmacology Target Classificationhttps://www.guidetopharmacology.org/targets.jsp
- Therapeutic Target Database (TTD)
- Drug Induced Liver Injury Rank (DILIrank) DatasetLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- Drugs and Lactation Database (LactMed)
- Japan Chemical Substance Dictionary (Nikkaji)
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.htmlAnatomical Therapeutic Chemical (ATC) classificationhttp://www.genome.jp/kegg-bin/get_htext?br08303.kegRisk category of Japanese OTC drugshttp://www.genome.jp/kegg-bin/get_htext?br08312.keg
- Metabolomics Workbench
- NCI Thesaurus (NCIt)LICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuseNCI Thesaurushttps://ncit.nci.nih.gov
- NLM RxNorm TerminologyLICENSEThe RxNorm Terminology is created by the National Library of Medicine (NLM) and is in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from NLM. Credit to the U.S. National Library of Medicine as the source is appreciated but not required. The full RxNorm dataset requires a free license.https://www.nlm.nih.gov/research/umls/rxnorm/docs/termsofservice.htmlbethanecholhttps://rxnav.nlm.nih.gov/id/rxnorm/19257
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/BETHANECHOLNORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- WHO Anatomical Therapeutic Chemical (ATC) ClassificationLICENSEUse of all or parts of the material requires reference to the WHO Collaborating Centre for Drug Statistics Methodology. Copying and distribution for commercial purposes is not allowed. Changing or manipulating the material is not allowed.https://www.whocc.no/copyright_disclaimer/
- PharmGKBLICENSEPharmGKB data are subject to the Creative Commons Attribution-ShareALike 4.0 license (https://creativecommons.org/licenses/by-sa/4.0/).https://www.pharmgkb.org/page/policiesbethanecholhttps://www.pharmgkb.org/chemical/PA448613
- PharosLICENSEData accessed from Pharos and TCRD is publicly available from the primary sources listed above. Please respect their individual licenses regarding proper use and redistribution.https://pharos.nih.gov/aboutbethanecholhttps://pharos.nih.gov/ligands/LZMDLZWYVFM7
- Wikidatabethanecholhttps://www.wikidata.org/wiki/Q419059
- WikipediaNickel arsenidehttps://en.wikipedia.org/wiki/Nickel_arsenideBethanecholhttps://en.wikipedia.org/wiki/Bethanechol
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlBethanecholhttps://www.ncbi.nlm.nih.gov/mesh/68018723Parasympathomimeticshttps://www.ncbi.nlm.nih.gov/mesh/68010277Muscarinic Agonistshttps://www.ncbi.nlm.nih.gov/mesh/68018721
- PubChem
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 390371312https://pubchem.ncbi.nlm.nih.gov/substance/390371312
- NCBI