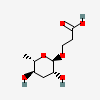
ascaroside C3
PubChem CID
44202063
Molecular Formula
Synonyms
- ascr#5
- ascaroside C3
- 1086696-26-5
- CHEBI:78829
- 3-[(2R,3R,5R,6S)-3,5-dihydroxy-6-methyloxan-2-yl]oxypropanoic acid
Molecular Weight
220.22 g/mol
Computed by PubChem 2.1 (PubChem release 2021.05.07)
Dates
- Create:2009-09-24
- Modify:2024-12-28
Description
Ascr#5 is an omega-hydroxy fatty acid ascaroside obtained by formal condensation of the alcoholic hydroxy group of 3-hydroxypropanoic acid with ascarylopyranose (the alpha anomer). A major component of the dauer pheromone of the nematode Caenorhabditis elegans, it synergises with ascr#2 and ascr#3 as a population-density signal to promote entry into an alternate larval stage, the nonfeeding and highly persistent dauer diapause. It has a role as a pheromone and a Caenorhabditis elegans metabolite. It is a monocarboxylic acid and an omega-hydroxy fatty acid ascaroside. It is functionally related to a 3-hydroxypropionic acid. It is a conjugate acid of an ascr#5(1-).
ascaroside C3 has been reported in Caenorhabditis elegans with data available.
Chemical Structure Depiction
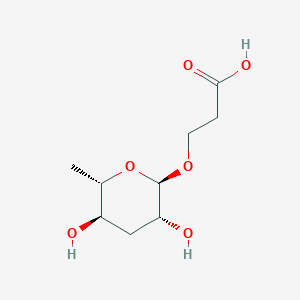
3-[(2R,3R,5R,6S)-3,5-dihydroxy-6-methyloxan-2-yl]oxypropanoic acid
Computed by LexiChem 2.6.6 (PubChem release 2019.06.18)
InChI=1S/C9H16O6/c1-5-6(10)4-7(11)9(15-5)14-3-2-8(12)13/h5-7,9-11H,2-4H2,1H3,(H,12,13)/t5-,6+,7+,9+/m0/s1
Computed by InChI 1.0.5 (PubChem release 2019.06.18)
RYYMJVGKZLQYPG-YYWONIAYSA-N
Computed by InChI 1.0.5 (PubChem release 2019.06.18)
C[C@H]1[C@@H](C[C@H]([C@@H](O1)OCCC(=O)O)O)O
Computed by OEChem 2.1.5 (PubChem release 2019.06.18)
C9H16O6
Computed by PubChem 2.1 (PubChem release 2019.06.18)
- ascr#5
- ascaroside C3
- 1086696-26-5
- CHEBI:78829
- 3-[(2R,3R,5R,6S)-3,5-dihydroxy-6-methyloxan-2-yl]oxypropanoic acid
- Propanoic acid, 3-[(3,6-dideoxy-alpha-L-arabino-hexopyranosyl)oxy]-
- 3-(((2R,3R,5R,6S)-3,5-Dihydroxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)propanoic acid
- MLS002460583
- CHEMBL1714874
- DTXSID001317806
- HMS2200P19
- 3-[(3,6-dideoxy-alpha-L-arabino-hexopyranosyl)oxy]propanoic acid
- HY-N6978
- AKOS040740708
- DA-60179
- MS-23222
- SMR001382724
- CS-0101359
- G16943
- 3-hydroxypropanoic acid 3-O-alpha-ascarylopyranoside
- Q27147980
- (-)-3-(3'R,5'R-dihydroxy-6'S-methyl-(2H)-tetrahydropyran-2'-yloxy)-propanoic acid
Property Name
Property Value
Reference
Property Name
Molecular Weight
Property Value
220.22 g/mol
Reference
Computed by PubChem 2.1 (PubChem release 2021.05.07)
Property Name
XLogP3-AA
Property Value
-1.1
Reference
Computed by XLogP3 3.0 (PubChem release 2019.06.18)
Property Name
Hydrogen Bond Donor Count
Property Value
3
Reference
Computed by Cactvs 3.4.6.11 (PubChem release 2019.06.18)
Property Name
Hydrogen Bond Acceptor Count
Property Value
6
Reference
Computed by Cactvs 3.4.6.11 (PubChem release 2019.06.18)
Property Name
Rotatable Bond Count
Property Value
4
Reference
Computed by Cactvs 3.4.6.11 (PubChem release 2019.06.18)
Property Name
Exact Mass
Property Value
220.09468823 Da
Reference
Computed by PubChem 2.1 (PubChem release 2021.05.07)
Property Name
Monoisotopic Mass
Property Value
220.09468823 Da
Reference
Computed by PubChem 2.1 (PubChem release 2021.05.07)
Property Name
Topological Polar Surface Area
Property Value
96.2Ų
Reference
Computed by Cactvs 3.4.6.11 (PubChem release 2019.06.18)
Property Name
Heavy Atom Count
Property Value
15
Reference
Computed by PubChem
Property Name
Formal Charge
Property Value
0
Reference
Computed by PubChem
Property Name
Complexity
Property Value
219
Reference
Computed by Cactvs 3.4.6.11 (PubChem release 2019.06.18)
Property Name
Isotope Atom Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Atom Stereocenter Count
Property Value
4
Reference
Computed by PubChem
Property Name
Undefined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Covalently-Bonded Unit Count
Property Value
1
Reference
Computed by PubChem
Property Name
Compound Is Canonicalized
Property Value
Yes
Reference
Computed by PubChem (release 2010.01.29)
Fatty Acyls [FA] -> Fatty acyl glycosides [FA13] -> Ascarosides [FA1304]
Follow these links to do a live 2D search or do a live 3D search for this compound, sorted by annotation score. This section is deprecated (see here for details), but these live search links provide equivalent functionality to the table that was previously shown here.
Same Connectivity Count
Same Parent, Connectivity Count
Same Parent, Exact Count
Mixtures, Components, and Neutralized Forms Count
Similar Compounds (2D)
Similar Conformers (3D)
WormJam Metabolites Local CSV for MetFrag | DOI:10.5281/zenodo.3403364
WormJam: A consensus C. elegans Metabolic Reconstruction and Metabolomics Community and Workshop Series, Worm, 6:2, e1373939, DOI:10.1080/21624054.2017.1373939
The LOTUS Initiative for Open Natural Products Research: frozen dataset union wikidata (with metadata) | DOI:10.5281/zenodo.5794106
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/
- EPA DSSToxAscaroside C3https://comptox.epa.gov/dashboard/DTXSID001317806CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- ChEBI
- LOTUS - the natural products occurrence databaseLICENSEThe code for LOTUS is released under the GNU General Public License v3.0.https://lotus.nprod.net/ascaroside C3https://www.wikidata.org/wiki/Q27147980LOTUS Treehttps://lotus.naturalproducts.net/
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.html
- ECI Group, LCSB, University of Luxembourgascr#5
- Natural Product Activity and Species Source (NPASS)3-[(2R,3R,5R,6S)-3,5-Dihydroxy-6-Methyloxan-2-Yl]Oxypropanoic Acidhttps://bidd.group/NPASS/compound.php?compoundID=NPC320032
- Japan Chemical Substance Dictionary (Nikkaji)
- LIPID MAPSAscaroside C3https://lipidmaps.org/databases/lmsd/LMFA13040005Lipid Classificationhttps://www.lipidmaps.org/
- Metabolomics Workbench
- Wikidata
- PubChem
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
CONTENTS