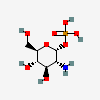
alpha-D-glucosamine 1-phosphate
PubChem CID
188960
Molecular Formula
Synonyms
- alpha-D-glucosamine 1-phosphate
- 2152-75-2
- Glucosamine 1-phosphate
- D-glucosamine 1-phosphate
- [(2R,3R,4R,5S,6R)-3-amino-4,5-dihydroxy-6-(hydroxymethyl)oxan-2-yl] dihydrogen phosphate
Molecular Weight
259.15 g/mol
Computed by PubChem 2.2 (PubChem release 2024.11.20)
Dates
- Create:2005-06-24
- Modify:2025-01-18
Description
Alpha-D-glucosamine 1-phosphate is a glucosamine phosphate. It has a role as an Escherichia coli metabolite. It is functionally related to an alpha-D-glucosamine. It is a conjugate acid of an alpha-D-glucosamine 1-phosphate(1-).
alpha-D-Glucosamine 1-phosphate is a metabolite found in or produced by Escherichia coli (strain K12, MG1655).
Glucosamine 1-phosphate has been reported in Daphnia pulex and Trypanosoma brucei with data available.
Chemical Structure Depiction
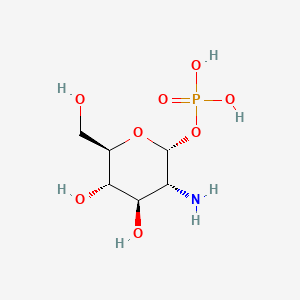
SVG Image

IUPAC Condensed
a-GlcN1P
IUPAC
phosphono 2-amino-2-deoxy-alpha-D-gluco-hexopyranoside
[(2R,3R,4R,5S,6R)-3-amino-4,5-dihydroxy-6-(hydroxymethyl)oxan-2-yl] dihydrogen phosphate
Computed by Lexichem TK 2.7.0 (PubChem release 2024.11.20)
InChI=1S/C6H14NO8P/c7-3-5(10)4(9)2(1-8)14-6(3)15-16(11,12)13/h2-6,8-10H,1,7H2,(H2,11,12,13)/t2-,3-,4-,5-,6-/m1/s1
Computed by InChI 1.07.0 (PubChem release 2024.11.20)
YMJBYRVFGYXULK-QZABAPFNSA-N
Computed by InChI 1.07.0 (PubChem release 2024.11.20)
C([C@@H]1[C@H]([C@@H]([C@H]([C@H](O1)OP(=O)(O)O)N)O)O)O
Computed by OEChem 2.3.0 (PubChem release 2024.12.12)
C6H14NO8P
Computed by PubChem 2.2 (PubChem release 2024.11.20)
glucosamine 1-phosphate
- alpha-D-glucosamine 1-phosphate
- 2152-75-2
- Glucosamine 1-phosphate
- D-glucosamine 1-phosphate
- [(2R,3R,4R,5S,6R)-3-amino-4,5-dihydroxy-6-(hydroxymethyl)oxan-2-yl] dihydrogen phosphate
- 2-amino-2-deoxy-1-O-phosphono-alpha-D-glucopyranose
- (2R,3R,4R,5S,6R)-3-Amino-4,5-dihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl dihydrogen phosphate
- 2-amino-2-deoxy-alpha-D-glucopyranose 1-(dihydrogen phosphate)
- alpha-D-Glucopyranose, 2-amino-2-deoxy-, 1-(dihydrogen phosphate)
- GP1
- BSG3MU7VAE
- SCHEMBL285055
- CHEBI:27625
- DTXSID70175855
- alpha -D-Glucosamine 1-phosphate
- MFCD00070181
- DB03111
- DB-225169
- HY-154871
- CS-0835704
- NS00069799
- A-D-GLUCOSAMINE 1-PHOSPHATE FREE ACID
- C06156
- G90968
- 2-amino-2-deoxy-alpha-d-glucopyranosyl phosphate
- J-014131
- .alpha.-D-Glucopyranose, 2-amino-2-deoxy-, 1-(dihydrogen phosphate)
- {[(2R,3R,4R,5S,6R)-3-amino-4,5-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}phosphonic acid
- (2R,3R,4R,5S,6R)-3-Amino-4,5-dihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yldihydrogenphosphate
Property Name
Property Value
Reference
Property Name
Molecular Weight
Property Value
259.15 g/mol
Reference
Computed by PubChem 2.2 (PubChem release 2024.11.20)
Property Name
XLogP3-AA
Property Value
-6.2
Reference
Computed by XLogP3 3.0 (PubChem release 2024.11.20)
Property Name
Hydrogen Bond Donor Count
Property Value
6
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2024.11.20)
Property Name
Hydrogen Bond Acceptor Count
Property Value
9
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2024.11.20)
Property Name
Rotatable Bond Count
Property Value
3
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2024.11.20)
Property Name
Exact Mass
Property Value
259.04570340 Da
Reference
Computed by PubChem 2.2 (PubChem release 2024.11.20)
Property Name
Monoisotopic Mass
Property Value
259.04570340 Da
Reference
Computed by PubChem 2.2 (PubChem release 2024.11.20)
Property Name
Topological Polar Surface Area
Property Value
163 Ų
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2024.11.20)
Property Name
Heavy Atom Count
Property Value
16
Reference
Computed by PubChem
Property Name
Formal Charge
Property Value
0
Reference
Computed by PubChem
Property Name
Complexity
Property Value
282
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2024.11.20)
Property Name
Isotope Atom Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Atom Stereocenter Count
Property Value
5
Reference
Computed by PubChem
Property Name
Undefined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Covalently-Bonded Unit Count
Property Value
1
Reference
Computed by PubChem
Property Name
Compound Is Canonicalized
Property Value
Yes
Reference
Computed by PubChem (release 2021.10.14)
153 Ų [M+H]+ [CCS Type: TW; Method: calibrated with polyalanine]
158.5 Ų [M+Na]+ [CCS Type: DT; Method: single field calibrated with Agilent tune mix (Agilent)]
Pharmaceuticals -> Listed in ZINC15
S55 | ZINC15PHARMA | Pharmaceuticals from ZINC15 | DOI:10.5281/zenodo.3247749
NIST Number
1171710
Instrument Type
IT/ion trap
Collision Energy
0
Spectrum Type
MS2
Precursor Type
[M+H]+
Precursor m/z
260.053
Total Peaks
116
m/z Top Peak
242
m/z 2nd Highest
224
m/z 3rd Highest
126
Thumbnail
NIST Number
1171777
Instrument Type
IT/ion trap
Collision Energy
0
Spectrum Type
MS2
Precursor Type
[M-H]-
Precursor m/z
258.0384
Total Peaks
28
m/z Top Peak
78.9
m/z 2nd Highest
240
m/z 3rd Highest
96.9
Thumbnail
Follow these links to do a live 2D search or do a live 3D search for this compound, sorted by annotation score. This section is deprecated (see here for details), but these live search links provide equivalent functionality to the table that was previously shown here.
Same Connectivity Count
Same Parent, Connectivity Count
Same Parent, Exact Count
Mixtures, Components, and Neutralized Forms Count
Similar Compounds (2D)
Similar Conformers (3D)
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=YMJBYRVFGYXULK-QZABAPFNSA-N
The LOTUS Initiative for Open Natural Products Research: frozen dataset union wikidata (with metadata) | DOI:10.5281/zenodo.5794106
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/α-D-Glucosamine 1-phosphatehttps://commonchemistry.cas.org/detail?cas_rn=2152-75-2
- ChemIDplusGlucosamine 1-phosphatehttps://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0002152752ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- EPA DSSToxalpha-D-Glucosamine 1-phosphatehttps://comptox.epa.gov/dashboard/DTXSID70175855CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingα-D-Glucosamine 1-phosphatehttps://gsrs.ncats.nih.gov/ginas/app/beta/substances/BSG3MU7VAE
- CCSbaseCCSbase Classificationhttps://ccsbase.net/
- ChEBIAlpha-D-glucosamine 1-phosphatehttps://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:27625
- E. coli Metabolome Database (ECMDB)
- LOTUS - the natural products occurrence databaseLICENSEThe code for LOTUS is released under the GNU General Public License v3.0.https://lotus.nprod.net/Glucosamine 1-phosphatehttps://www.wikidata.org/wiki/Q27094063LOTUS Treehttps://lotus.naturalproducts.net/
- Yeast Metabolome Database (YMDB)alpha-D-glucosamine 1-phosphatehttps://www.ymdb.ca/compounds/YMDB00754
- DrugBankLICENSECreative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode)https://www.drugbank.ca/legal/terms_of_useGlucosamine 1-Phosphatehttps://www.drugbank.ca/drugs/DB03111
- Japan Chemical Substance Dictionary (Nikkaji)
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.html
- Natural Product Activity and Species Source (NPASS)
- Metabolomics Workbenchalpha-D-glucosamine 1-phosphatehttps://www.metabolomicsworkbench.org/data/StructureData.php?RegNo=51395
- NIST Mass Spectrometry Data CenterLICENSEData covered by the Standard Reference Data Act of 1968 as amended.https://www.nist.gov/srd/public-lawD-Glucosamine 1-phosphatehttp://www.nist.gov/srd/nist1a.cfm
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/Glucosamine 1-phosphateNORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- Protein Data Bank in Europe (PDBe)
- RCSB Protein Data Bank (RCSB PDB)LICENSEData files contained in the PDB archive (ftp://ftp.wwpdb.org) are free of all copyright restrictions and made fully and freely available for both non-commercial and commercial use. Users of the data should attribute the original authors of that structural data.https://www.rcsb.org/pages/policies
- Springer Nature
- Wikidataalpha-D-glucosamine 1-phosphatehttps://www.wikidata.org/wiki/Q27094063
- PubChem
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlglucosamine 1-phosphatehttps://www.ncbi.nlm.nih.gov/mesh/67038515
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 397035889https://pubchem.ncbi.nlm.nih.gov/substance/397035889
- NCBI
CONTENTS