Zoledronate Disodium
- Zoledronate disodium hydrate
- Zoledronate disodium tetrahydrate
- 165800-07-7
- Zoledronate disodium
- Zoledronate disodium [USAN]
- Create:2008-01-22
- Modify:2025-01-11
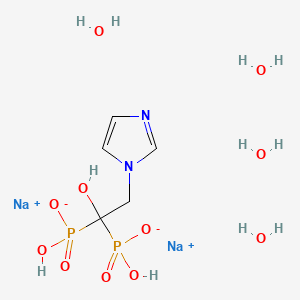
- 2-(imidazol-1-yl)-1-hydroxyethylidene-1,1-bisphosphonic acid
- CGP 42'446
- CGP 42446
- CGP 42446A
- CGP-42'446
- CGP-42446
- CGP42'446
- CGP42446
- zoledronate
- zoledronic acid
- zoledronic acid anhydrous
- Zometa
- Zoledronate disodium hydrate
- Zoledronate disodium tetrahydrate
- 165800-07-7
- Zoledronate disodium
- Zoledronate disodium [USAN]
- UNII-7D7GS1SA24
- 7D7GS1SA24
- Zoledronic Acid Disodium Salt Tetrahydrate
- CGP 42446A
- Zoledronate disodium (USAN)
- ZOLEDRONATE DISODIUM [MART.]
- ZOLEDRONATE DISODIUM [WHO-DD]
- Zoledronic Acid, Disodium Salt, Tetrahydrate
- Disodium dihydrogen (1-hydroxy-2-imidazol-1-ylethylidene)diphosphonate, tetrahydrate
- Phosphonic acid, (1-hydroxy-2-(1H-imidazol-1-yl)ethylidene)bis-, disodium salt tetrahydrate
- disodium;hydroxy-[1-hydroxy-1-[hydroxy(oxido)phosphoryl]-2-imidazol-1-ylethyl]phosphinate;tetrahydrate
- ZOLEDRONIC ACID DISODIUM SALT TETRAHYDRATE [MI]
- ZOLEDRONATE DISODIUM (MART.)
- Phosphonic acid, (1-hydroxy-2-(1H-imidazol-1-yl)ethylidene)bis-, disodium salt, tetrahydrate
- DTXSID80157167
- CGP 42446
- PHOSPHONIC ACID, P,P'-(1-HYDROXY-2-(1H-IMIDAZOL-1-YL)ETHYLIDENE)BIS-, SODIUM SALT (1:2)
- CHEMBL2103912
- DTXCID3079658
- IEJZOPBVBXAOBH-UHFFFAOYSA-L
- BCP24274
- CGP42446
- CGP42'446
- Zoledronate disodium salt tetrahydrate
- AKOS025401592
- CGP 42'446
- CGP-42'446
- AC-22574
- D06378
- Q27268106
- 158859-43-9
◉ Summary of Use during Lactation
Because no information is available on the use of zoledronic acid during breastfeeding, an alternate drug may be preferred, especially while nursing a newborn or preterm infant. However, absorption of zoledronic acid by a breastfed infant is unlikely.
◉ Effects in Breastfed Infants
Relevant published information was not found as of the revision date.
◉ Effects on Lactation and Breastmilk
Relevant published information was not found as of the revision date.
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=IEJZOPBVBXAOBH-UHFFFAOYSA-L
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.html
- ChemIDplusZoledronate disodium [USAN]https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0165800077ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingZOLEDRONATE DISODIUMhttps://gsrs.ncats.nih.gov/ginas/app/beta/substances/7D7GS1SA24
- Drugs and Lactation Database (LactMed)Zoledronic Acidhttps://www.ncbi.nlm.nih.gov/books/n/lactmed/LM722/
- EU Clinical Trials Register
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.htmlAnatomical Therapeutic Chemical (ATC) classificationhttp://www.genome.jp/kegg-bin/get_htext?br08303.kegTarget-based classification of drugshttp://www.genome.jp/kegg-bin/get_htext?br08310.keg
- NCI Thesaurus (NCIt)LICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuseNCI Thesaurushttps://ncit.nci.nih.gov
- Wikidatazoledronate disodium tetrahydratehttps://www.wikidata.org/wiki/Q27268106
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlZoledronic Acidhttps://www.ncbi.nlm.nih.gov/mesh/2027935Bone Density Conservation Agentshttps://www.ncbi.nlm.nih.gov/mesh/68050071
- PubChem
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 390419215https://pubchem.ncbi.nlm.nih.gov/substance/390419215

 CID 68740 (Zoledronate)
CID 68740 (Zoledronate) CID 5360545 (Sodium)
CID 5360545 (Sodium) CID 962 (Water)
CID 962 (Water)