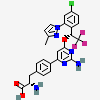
Telotristat
- Telotristat
- 1033805-28-5
- LP-778902
- UNII-381V4FCV2Z
- 381V4FCV2Z
- Create:2008-11-17
- Modify:2025-01-18
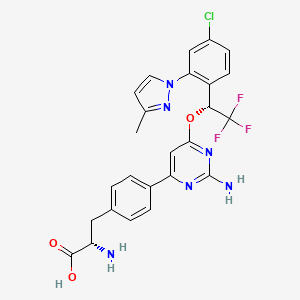
- LX1606
- telotristat
- telotristat etiprate
- Telotristat
- 1033805-28-5
- LP-778902
- UNII-381V4FCV2Z
- 381V4FCV2Z
- LP 778902
- DTXSID60145805
- (2S)-2-amino-3-(4-{2-amino-6-[(1R)-1-[4-chloro-2-(3-methyl-1H-pyrazol-1-yl)phenyl]-2,2,2-trifluoroethoxy]pyrimidin-4-yl}phenyl)propanoic acid
- (2S)-2-amino-3-[4-[2-amino-6-[(1R)-1-[4-chloro-2-(3-methylpyrazol-1-yl)phenyl]-2,2,2-trifluoroethoxy]pyrimidin-4-yl]phenyl]propanoic acid
- (S)-2-amino-3-(4-(2-amino-6-((R)-1-(4-chloro-2-(3-methyl-1H-pyrazol-1-yl)phenyl)-2,2,2-trifluoroethoxy)pyrimidin-4-yl)phenyl)propanoic acid
- (2S)-2-aMino-3-[4-[2-aMino-6-[(1R)-1-[4-chloro-2-(3-Methylpyrazol-1-yl)phenyl]-2,2,2-trifluoroethoxy]pyriMidin-4-yl]phenyl]propanoic acid, Telotristat
- 2S)-2-AMINO-3-(4-(2-AMINO-6-(((1R)-1-(4-CHLORO-2-(3-METHYL-1H-PYRAZOL-1-YL)PHENYL)-2,2,2-TRIFLUOROETHYL)OXY)PYRIMIDIN-4-YL)PHENYL)PROPANOIC ACID
- Telotristat [USAN]
- Telotristat [USAN:INN]
- telotristatum
- Telotristat (Standard)
- TELOTRISTAT [MI]
- TELOTRISTAT [INN]
- Telotristat (USAN/INN)
- Telotristat, LP778902
- TELOTRISTAT [WHO-DD]
- SCHEMBL612353
- CHEMBL2103855
- DTXCID4068296
- A16AX15
- CHEBI:177736
- HY-13055BR
- EX-A1620
- BDBM50145648
- HY-13055B
- MFCD20528907
- AKOS027338710
- DB14218
- LX-3033
- NCGC00485953-01
- AC-35764
- MS-30026
- D09973
- F86418
- BRD-K63178091-001-01-1
- Q27256725
- (2S)-2-amino-3-[4-[2-amino-6-[(1R)-1-[4-chloro-2-(3-methylpyrazol-1-yl)phenyl]-2,2,2-triluoroethoxy]pyrimidin-4-yl]phenyl]propanoic acid
- 4-(2-AMINO-6-((1R)-1-(4-CHLORO-2-(3-METHYL-1H-PYRAZOL-1-YL)PHENYL)-2,2,2- TRIFLUOROETHOXY)PRYRIMIDIN-4-YL)-L-PHENYLALANINE
- 4-(2-AMINO-6-((1R)-1-(4-CHLORO-2-(3-METHYL-1H-PYRAZOL-1-YL)PHENYL)-2,2,2-TRIFLUOROETHOXY)PRYRIMIDIN-4-YL)-L-PHENYLALANINE
- L-PHENYLALANINE, 4-(2-AMINO-6-((1R)-1-(4-CHLORO-2-(3-METHYL-1H-PYRAZOL-1-YL)PHENYL)- 2,2,2-TRIFLUOROETHOXY)-4-PRYRIMIDINYL)-
- L-PHENYLALANINE, 4-(2-AMINO-6-((1R)-1-(4-CHLORO-2-(3-METHYL-1H-PYRAZOL-1-YL)PHENYL)-2,2,2-TRIFLUOROETHOXY)-4-PRYRIMIDINYL)-
- Telotristat; L-Phenylalanine, 4-[2-amino-6-[(1R)-1-[4-chloro-2-(3-methyl-1H-pyrazol-1-yl)phenyl]-2,2,2-trifluoroethoxy]-4-pyrimidinyl]-; 4-[2-Amino-6-[(1R)-1-[4-chloro-2-(3-methyl-1H-pyrazol-1-yl)phenyl]-2,2,2-trifluoroethoxy]-4-pyrimidinyl]-L-phenylalanine; (2S)-2-Am
Telotristat Ethyl (active moiety of)
Telotristat Etiprate (is active moiety of)
In small clinical trials of telotristat in patients with neuroendocrine tumors and symptomatic diarrhea despite stable doses of somatostatin analogues, transient, asymptomatic and mild serum elevations of ALT occurred in 4% to 5% and of GGT in 6% to 9% of treated subjects. Apparent liver injury with jaundice was not observed in the several prelicensure clinical trials and has not been reported in the limited clinical experience with this agent since its approval.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=NCLGDOBQAWBXRA-PGRDOPGGSA-N
- ChEBI
- DrugBankLICENSECreative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode)https://www.drugbank.ca/legal/terms_of_useTelotristathttps://www.drugbank.ca/drugs/DB14218
- FDA Pharm ClassesLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingFDA Pharmacological Classificationhttps://www.fda.gov/ForIndustry/DataStandards/StructuredProductLabeling/ucm162549.htm
- LiverTox
- NCI Thesaurus (NCIt)LICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuseNCI Thesaurushttps://ncit.nci.nih.gov
- Open TargetsLICENSEDatasets generated by the Open Targets Platform are freely available for download.https://platform-docs.opentargets.org/licence
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.htmlChEMBL Protein Target Treehttps://www.ebi.ac.uk/chembl/g/#browse/targets
- ChemIDplusTelotristat [USAN:INN]https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=1033805285ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- EPA DSSToxCompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- ClinicalTrials.govLICENSEThe ClinicalTrials.gov data carry an international copyright outside the United States and its Territories or Possessions. Some ClinicalTrials.gov data may be subject to the copyright of third parties; you should consult these entities for any additional terms of use.https://clinicaltrials.gov/ct2/about-site/terms-conditions#Use
- Drug Gene Interaction database (DGIdb)LICENSEThe data used in DGIdb is all open access and where possible made available as raw data dumps in the downloads section.http://www.dgidb.org/downloadsTELOTRISTAThttps://www.dgidb.org/drugs/rxcui:1872382
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.htmlAnatomical Therapeutic Chemical (ATC) classificationhttp://www.genome.jp/kegg-bin/get_htext?br08303.kegTarget-based classification of drugshttp://www.genome.jp/kegg-bin/get_htext?br08310.keg
- Metabolomics Workbench
- NLM RxNorm TerminologyLICENSEThe RxNorm Terminology is created by the National Library of Medicine (NLM) and is in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from NLM. Credit to the U.S. National Library of Medicine as the source is appreciated but not required. The full RxNorm dataset requires a free license.https://www.nlm.nih.gov/research/umls/rxnorm/docs/termsofservice.htmltelotristathttps://rxnav.nlm.nih.gov/id/rxnorm/1872382
- PharosLICENSEData accessed from Pharos and TCRD is publicly available from the primary sources listed above. Please respect their individual licenses regarding proper use and redistribution.https://pharos.nih.gov/aboutTelotristathttps://pharos.nih.gov/ligands/43P18J3UMLMT
- Springer Nature
- WHO Anatomical Therapeutic Chemical (ATC) ClassificationLICENSEUse of all or parts of the material requires reference to the WHO Collaborating Centre for Drug Statistics Methodology. Copying and distribution for commercial purposes is not allowed. Changing or manipulating the material is not allowed.https://www.whocc.no/copyright_disclaimer/
- Wikidatatelotristathttps://www.wikidata.org/wiki/Q27256725
- PubChemPFAS and Fluorinated Compounds in PubChemhttps://gitlab.com/uniluxembourg/lcsb/eci/pubchem-docs/-/raw/main/pfas-tree/PFAS_Tree.pdf?inline=false
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmltelotristathttps://www.ncbi.nlm.nih.gov/mesh/2003044
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 402579904https://pubchem.ncbi.nlm.nih.gov/substance/402579904
- NCBI