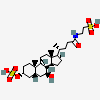
Tauroursodeoxycholate-3-sulfate
PubChem CID
101931089
Molecular Formula
Synonyms
- tauroursodeoxycholate-3-sulfate
- Q63393417
Molecular Weight
579.8 g/mol
Computed by PubChem 2.2 (PubChem release 2024.11.20)
Dates
- Create:2015-12-18
- Modify:2025-01-18
Chemical Structure Depiction
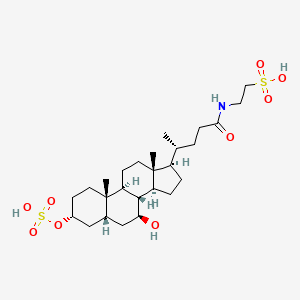
2-[[(4R)-4-[(3R,5R,7S,8R,9S,10S,13R,14S,17R)-7-hydroxy-10,13-dimethyl-3-sulfooxy-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl]pentanoyl]amino]ethanesulfonic acid
Computed by Lexichem TK 2.7.0 (PubChem release 2024.11.20)
InChI=1S/C26H45NO9S2/c1-16(4-7-23(29)27-12-13-37(30,31)32)19-5-6-20-24-21(9-11-26(19,20)3)25(2)10-8-18(36-38(33,34)35)14-17(25)15-22(24)28/h16-22,24,28H,4-15H2,1-3H3,(H,27,29)(H,30,31,32)(H,33,34,35)/t16-,17+,18-,19-,20+,21+,22+,24+,25+,26-/m1/s1
Computed by InChI 1.07.0 (PubChem release 2024.11.20)
GLVWZDCWCRWVFM-LBSADWJPSA-N
Computed by InChI 1.07.0 (PubChem release 2024.11.20)
C[C@H](CCC(=O)NCCS(=O)(=O)O)[C@H]1CC[C@@H]2[C@@]1(CC[C@H]3[C@H]2[C@H](C[C@H]4[C@@]3(CC[C@H](C4)OS(=O)(=O)O)C)O)C
Computed by OEChem 2.3.0 (PubChem release 2024.12.12)
C26H45NO9S2
Computed by PubChem 2.2 (PubChem release 2024.11.20)
Property Name
Property Value
Reference
Property Name
Molecular Weight
Property Value
579.8 g/mol
Reference
Computed by PubChem 2.2 (PubChem release 2024.11.20)
Property Name
XLogP3-AA
Property Value
3.2
Reference
Computed by XLogP3 3.0 (PubChem release 2024.11.20)
Property Name
Hydrogen Bond Donor Count
Property Value
4
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2024.11.20)
Property Name
Hydrogen Bond Acceptor Count
Property Value
9
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2024.11.20)
Property Name
Rotatable Bond Count
Property Value
9
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2024.11.20)
Property Name
Exact Mass
Property Value
579.25357436 Da
Reference
Computed by PubChem 2.2 (PubChem release 2024.11.20)
Property Name
Monoisotopic Mass
Property Value
579.25357436 Da
Reference
Computed by PubChem 2.2 (PubChem release 2024.11.20)
Property Name
Topological Polar Surface Area
Property Value
184 Ų
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2024.11.20)
Property Name
Heavy Atom Count
Property Value
38
Reference
Computed by PubChem
Property Name
Formal Charge
Property Value
0
Reference
Computed by PubChem
Property Name
Complexity
Property Value
1080
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2024.11.20)
Property Name
Isotope Atom Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Atom Stereocenter Count
Property Value
10
Reference
Computed by PubChem
Property Name
Undefined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Covalently-Bonded Unit Count
Property Value
1
Reference
Computed by PubChem
Property Name
Compound Is Canonicalized
Property Value
Yes
Reference
Computed by PubChem (release 2015.09.10)
Accession ID
Authors
Takashi Iida, Department of Chemistry, College of Humanities and Sciences, Nihon University
Instrument
JMS-T100LP, JEOL
Instrument Type
LC-ESI-TOF
MS Level
MS
Ionization Mode
NEGATIVE
Ionization
ESI
Top 5 Peaks
288.61 999
290.12 35
License
CC BY
Accession ID
Authors
Takashi Iida, Department of Chemistry, College of Humanities and Sciences, Nihon University
Instrument
JMS-T100LP, JEOL
Instrument Type
LC-ESI-TOF
MS Level
MS
Ionization Mode
NEGATIVE
Ionization
ESI
Top 5 Peaks
288.61 999
480.27 68
68.99 37
290.11 35
61.98 20
License
CC BY
MoNA ID
MS Category
Experimental
MS Type
Other
MS Level
MS2
Precursor Type
[2M+Na]+
Precursor m/z
1181.5
Instrument
qTof
Ionization Mode
positive
Top 5 Peaks
464.282288 100
465.283752 37.75
504.275177 31.96
522.285034 29.67
999.602661 11.51
MoNA ID
MS Category
Experimental
MS Type
Other
MS Level
MS2
Precursor Type
[M+Na]+
Precursor m/z
602.243
Instrument
qTof
Ionization Mode
positive
Top 5 Peaks
486.263428 100
148.003799 93.45
502.229126 31.11
487.268982 27.48
503.233795 11.99
Follow these links to do a live 2D search or do a live 3D search for this compound, sorted by annotation score. This section is deprecated (see here for details), but these live search links provide equivalent functionality to the table that was previously shown here.
Same Connectivity Count
Same Stereo Count
Same Isotope Count
Same Parent, Connectivity Count
Same Parent, Stereo Count
Same Parent, Isotope Count
Same Parent, Exact Count
Mixtures, Components, and Neutralized Forms Count
Similar Compounds (2D)
Similar Conformers (3D)
- Japan Chemical Substance Dictionary (Nikkaji)
- MassBank EuropeTauroursodeoxycholate-3-sulfatehttps://massbank.eu/MassBank/Result.jsp?inchikey=GLVWZDCWCRWVFM-LBSADWJPSA-N
- MassBank of North America (MoNA)LICENSEThe content of the MoNA database is licensed under CC BY 4.0.https://mona.fiehnlab.ucdavis.edu/documentation/license
- Metabolomics WorkbenchTauroursodeoxycholate-3-sulfatehttps://www.metabolomicsworkbench.org/data/StructureData.php?RegNo=199540
- Wikidata2-[[(4R)-4-[(3R,5R,7S,8R,9S,10S,13R,14S,17R)-7-hydroxy-10,13-dimethyl-3-sulfooxy-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl]pentanoyl]amino]ethanesulfonic acidhttps://www.wikidata.org/wiki/Q63393417
- PubChem
CONTENTS