Tantalum trialuminide
PubChem CID
6336851
Molecular Formula
Synonyms
- tantalum trialuminide
- Q10860151
Molecular Weight
261.8925 g/mol
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Component Compounds
Dates
- Create:2005-08-08
- Modify:2025-01-11
Description
L739: ATSDR - Agency for Toxic Substances and Disease Registry (2008). Toxicological profile for aluminum. U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). http://www.atsdr.cdc.gov/toxprofiles/tp22.html
L740: Wikipedia. Aluminium. Last Updated 16 June 2009. http://en.wikipedia.org/wiki/Aluminum
Chemical Structure Depiction
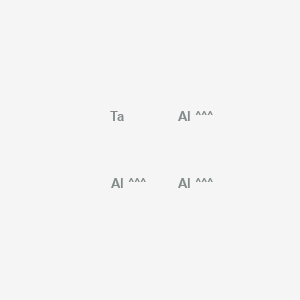
Conformer generation is disallowed since MMFF94s unsupported element, MMFF94s unsupported atom valence, mixture or salt
InChI=1S/3Al.Ta
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
LJVJDEGCVHBSKZ-UHFFFAOYSA-N
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
[Al].[Al].[Al].[Ta]
Computed by OEChem 2.3.0 (PubChem release 2024.12.12)
Al3Ta
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Property Value
Reference
Property Name
Molecular Weight
Property Value
261.8925 g/mol
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Donor Count
Property Value
0
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Acceptor Count
Property Value
0
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Rotatable Bond Count
Property Value
0
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Exact Mass
Property Value
261.89261 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Monoisotopic Mass
Property Value
261.89261 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Topological Polar Surface Area
Property Value
0 Ų
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Heavy Atom Count
Property Value
4
Reference
Computed by PubChem
Property Name
Formal Charge
Property Value
0
Reference
Computed by PubChem
Property Name
Complexity
Property Value
0
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Isotope Atom Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Covalently-Bonded Unit Count
Property Value
4
Reference
Computed by PubChem
Property Name
Compound Is Canonicalized
Property Value
Yes
Reference
Computed by PubChem (release 2021.10.14)
Follow these links to do a live 2D search or do a live 3D search for this compound, sorted by annotation score. This section is deprecated (see here for details), but these live search links provide equivalent functionality to the table that was previously shown here.
Mixtures, Components, and Neutralized Forms Count
Similar Compounds (2D)
Similar Conformers (3D)
Same Count
Aluminum is poorly absorbed following either oral or inhalation exposure and is essentially not absorbed dermally. The bioavailability of aluminum is strongly influenced by the aluminum compound and the presence of dietary constituents which can complex with aluminum and enhance or inhibit its absorption. Aluminum binds to various ligands in the blood and distributes to every organ, with highest concentrations found in bone and lung tissues. In living organisms, aluminum is believed to exist in four different forms: as free ions, as low-molecular-weight complexes, as physically bound macromolecular complexes, and as covalently bound macromolecular complexes. Absorbed aluminum is excreted principally in the urine and, to a lesser extent, in the bile, while unabsorbed aluminum is excreted in the faeces. (L739)
L739: ATSDR - Agency for Toxic Substances and Disease Registry (2008). Toxicological profile for aluminum. U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). http://www.atsdr.cdc.gov/toxprofiles/tp22.html
The main target organs of aluminum are the central nervous system and bone. Aluminum binds with dietary phosphorus and impairs gastrointestinal absorption of phosphorus. The decreased phosphate body burden results in osteomalacia (softening of the bones due to defective bone mineralization) and rickets. Aluminum's neurotoxicity is believed to involve several mechanisms. Changes in cytoskeletal protein functions as a results of altered phosphorylation, proteolysis, transport, and synthesis are believed to be one cause. Aluminum may induce neurobehavioral effects by affecting permeability of the blood-brain barrier, cholinergic activity, signal transduction pathways, lipid peroxidation, and impair neuronal glutamate nitric oxide-cyclic GMP pathway, as well as interfere with metabolism of essential trace elements because of similar coordination chemistries and consequent competitive interactions. It has been suggested that aluminum's interaction with estrogen receptors increases the expression of estrogen-related genes and thereby contributes to the progression of breast cancer (A235), but studies have not been able to establish a clear link between aluminum and increased risk of breast cancer (A15468). Certain aluminum salts induce immune responses by activating inflammasomes. (L739, A235, A236)
A15468: Willhite CC, Karyakina NA, Yokel RA, Yenugadhati N, Wisniewski TM, Arnold IM, Momoli F, Krewski D: Systematic review of potential health risks posed by pharmaceutical, occupational and consumer exposures to metallic and nanoscale aluminum, aluminum oxides, aluminum hydroxide and its soluble salts. Crit Rev Toxicol. 2014 Oct;44 Suppl 4:1-80. doi: 10.3109/10408444.2014.934439. PMID:25233067
A235: Darbre PD: Metalloestrogens: an emerging class of inorganic xenoestrogens with potential to add to the oestrogenic burden of the human breast. J Appl Toxicol. 2006 May-Jun;26(3):191-7. PMID:16489580
A236: Aimanianda V, Haensler J, Lacroix-Desmazes S, Kaveri SV, Bayry J: Novel cellular and molecular mechanisms of induction of immune responses by aluminum adjuvants. Trends Pharmacol Sci. 2009 Jun;30(6):287-95. doi: 10.1016/j.tips.2009.03.005. Epub 2009 May 11. PMID:19439372
L739: ATSDR - Agency for Toxic Substances and Disease Registry (2008). Toxicological profile for aluminum. U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). http://www.atsdr.cdc.gov/toxprofiles/tp22.html
Carcinogen Classification
Not listed by IARC. IARC classified aluminum production as carcinogenic to humans (Group 1), but did not implicate aluminum itself as a human carcinogen. (L135) A link between use of aluminum-containing antiperspirants and increased risk of breast cancer has been proposed (A235), but studies have not been able to establish a clear link (A15468).
Aluminum targets the nervous system and causes decreased nervous system performance and is associated with altered function of the blood-brain barrier. The accumulation of aluminum in the body may cause bone or brain diseases. High levels of aluminum have been linked to Alzheimer's disease. A small percentage of people are allergic to aluminium and experience contact dermatitis, digestive disorders, vomiting or other symptoms upon contact or ingestion of products containing aluminium. (L739, L740)
L739: ATSDR - Agency for Toxic Substances and Disease Registry (2008). Toxicological profile for aluminum. U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). http://www.atsdr.cdc.gov/toxprofiles/tp22.html
L740: Wikipedia. Aluminium. Last Updated 16 June 2009. http://en.wikipedia.org/wiki/Aluminum
Oral (L739) ; inhalation (L739)
L739: ATSDR - Agency for Toxic Substances and Disease Registry (2008). Toxicological profile for aluminum. U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). http://www.atsdr.cdc.gov/toxprofiles/tp22.html
L739: ATSDR - Agency for Toxic Substances and Disease Registry (2008). Toxicological profile for aluminum. U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). http://www.atsdr.cdc.gov/toxprofiles/tp22.html
L740: Wikipedia. Aluminium. Last Updated 16 June 2009. http://en.wikipedia.org/wiki/Aluminum
Intermediate Oral: 1.0 mg/kg/day (L134)
Chronic Oral: 1.0 mg/kg/day (L134)
L134: ATSDR - Agency for Toxic Substances and Disease Registry (2001). Minimal Risk Levels (MRLs) for Hazardous Substances. U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). http://www.atsdr.cdc.gov/mrls/
- Toxin and Toxin Target Database (T3DB)LICENSET3DB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (T3DB) and the original publication.http://www.t3db.ca/downloadsTantalum(III) aluminidehttp://www.t3db.ca/toxins/T3D1542
- Wikidatatantalum trialuminidehttps://www.wikidata.org/wiki/Q10860151
- WikipediaTantalum trialuminidehttps://en.wikipedia.org/wiki/Tantalum_trialuminide
- PubChem
CONTENTS

 CID 5359268 (Aluminum)
CID 5359268 (Aluminum) CID 23956 (Tantalum)
CID 23956 (Tantalum)