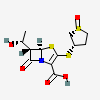
Sulopenem
- Sulopenem
- 120788-07-0
- CP-70429
- CP-70,429
- (5R,6S)-6-[(1R)-1-hydroxyethyl]-7-oxo-3-[(1R,3S)-1-oxothiolan-3-yl]sulfanyl-4-thia-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid
- Create:2006-10-25
- Modify:2025-01-18
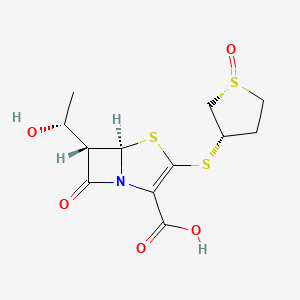
- CP 70429
- CP-70,429
- CP-70429
- sulopenem
- Sulopenem
- 120788-07-0
- CP-70429
- CP-70,429
- (5R,6S)-6-[(1R)-1-hydroxyethyl]-7-oxo-3-[(1R,3S)-1-oxothiolan-3-yl]sulfanyl-4-thia-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid
- XX514BJ1XW
- CP 70429
- SULOPENEM [INN]
- Sulopenem (USAN/INN)
- SULOPENEM [USAN]
- SCHEMBL2256409
- CHEMBL1908305
- Sulopenem, >=98% (HPLC)
- GTPL10862
- DTXSID20869656
- (5R,6S)-6-[(1R)-1-Hydroxyethyl]-7-oxo-3-[[(3S)-tetrahydro-3-thienyl]thio]-4-thia-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid, (R)-S-oxide
- EX-A4518
- AKOS040745084
- CP-65207-S
- CP70429
- DB15284
- AC-33244
- MS-25396
- CP-70429?
- HY-105284
- CS-0025618
- Orlynvah (sulopenem etzadroxil and probenecid)
- D05969
- G12694
- Q27294038
- (5R,6S)-6-((1R)-1-HYDROXYETHYL)-7-OXO-3-(((1R,3S)-1-OXOTETRAHYDRO-1H-1.LAMBA.(SUP 4)-THIOPHEN-3-YL)SULFANYL)-4-THIA-1-AZABICYCLO(3.2.0)HEPT-2-ENE-2-CARBOXYLIC ACID
- 4-THIA-1-AZABICYCLO(3.2.0)HEPT-2-ENE-2-CARBOXYLIC ACID, 6-((1R)-1-HYDROXYETHYL)-7-OXO-3-(((1R,3S)-TETRAHYDRO-1-OXIDO-3-THIENYL)THIO)-, (5R,6S)-
- 4-THIA-1-AZABICYCLO(3.2.0)HEPT-2-ENE-2-CARBOXYLIC ACID, 6-(1-HYDROXYETHYL)-7-OXO-3-((TETRAHYDRO-3-THIENYL)THIO)-, S-OXIDE, (5R-(3(1R*,3S*),5.ALPHA.,6.ALPHA.(R*)))-
- Sulopenem; 4-Thia-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid, 6-[(1R)-1-hydroxyethyl]-7-oxo-3-[[(1R,3S)-tetrahydro-1-oxido-3-thienyl]thio]-, (5R,6S)-; 4-Thia-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid, 6-(1-hydroxyethyl)-7-oxo-3-[(tetrahydro-3-thienyl)thio
◉ Summary of Use during Lactation
No information is available on the clinical use of sulopenem during breastfeeding. Its excretion into breastmilk is likely similar to that of imipenem and meropenem, which produce low levels in milk that are not expected to cause adverse effects in breastfed infants. Limited information indicates that maternal doses of probenecid up to 2 grams daily produce low levels in milk and would not be expected to cause any adverse effects in breastfed infants, especially if the infant is older than 2 months. Occasionally disruption of the infant's gastrointestinal flora, resulting in diarrhea or thrush has been reported with beta-lactams, but these effects have not been adequately evaluated. Sulopenem-probenecid is acceptable in nursing mothers.
◉ Effects in Breastfed Infants
Relevant published information was not found as of the revision date.
◉ Effects on Lactation and Breastmilk
Relevant published information was not found as of the revision date.
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=FLSUCZWOEMTFAQ-PRBGKLEPSA-N
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.html
- ClinicalTrials.govLICENSEThe ClinicalTrials.gov data carry an international copyright outside the United States and its Territories or Possessions. Some ClinicalTrials.gov data may be subject to the copyright of third parties; you should consult these entities for any additional terms of use.https://clinicaltrials.gov/ct2/about-site/terms-conditions#Use
- DrugBankLICENSECreative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode)https://www.drugbank.ca/legal/terms_of_use
- NCI Thesaurus (NCIt)LICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuseNCI Thesaurushttps://ncit.nci.nih.gov
- Open TargetsLICENSEDatasets generated by the Open Targets Platform are freely available for download.https://platform-docs.opentargets.org/licence
- EPA DSSToxCompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- Drugs and Lactation Database (LactMed)Sulopenem and Probenecidhttps://www.ncbi.nlm.nih.gov/books/n/lactmed/sulopenem_probeneci/
- EU Clinical Trials Register
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.htmlAntiinfectiveshttp://www.genome.jp/kegg-bin/get_htext?br08307.keg
- Metabolomics Workbench
- Therapeutic Target Database (TTD)
- Thieme ChemistryLICENSEThe Thieme Chemistry contribution within PubChem is provided under a CC-BY-NC-ND 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc-nd/4.0/
- Wikidata
- Wikipedia5-Chloro-DMThttps://en.wikipedia.org/wiki/5-Chloro-DMT
- PubChem
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.html
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 403232805https://pubchem.ncbi.nlm.nih.gov/substance/403232805