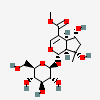
Shanzhiside methyl ester
- Shanzhiside methyl ester
- 64421-28-9
- SHANZHISIDE METHYLESTER
- HANZHISIDE METHYL ESTER
- methyl (1S,4aS,5R,7S,7aS)-5,7-dihydroxy-7-methyl-1-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-4a,5,6,7a-tetrahydro-1H-cyclopenta[c]pyran-4-carboxylate
- Create:2007-02-08
- Modify:2025-01-04
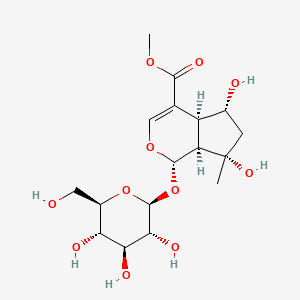
- Shanzhiside methyl ester
- 64421-28-9
- SHANZHISIDE METHYLESTER
- HANZHISIDE METHYL ESTER
- methyl (1S,4aS,5R,7S,7aS)-5,7-dihydroxy-7-methyl-1-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-4a,5,6,7a-tetrahydro-1H-cyclopenta[c]pyran-4-carboxylate
- Methyl (1S,4aS,5R,7S,7aS)-5,7-dihydroxy-7-methyl-1-(((2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl)oxy)-1,4a,5,6,7,7a-hexahydrocyclopenta[c]pyran-4-carboxylate
- Shanzhisidmethylester
- MFCD00017416
- MEGxp0_000430
- CHEMBL1081414
- ACon0_000910
- ACon1_000460
- HY-N0630R
- CHEBI:193584
- HY-N0630
- Shanzhiside methyl ester (Standard)
- s9307
- AKOS030573532
- CCG-268713
- NCGC00169057-01
- NCGC00169057-02
- AC-34703
- MS-26955
- CS-0009621
- NS00097692
- F17713
- BRD-K61757038-001-01-0
- Z3247556297
- (1S,4aS,5R,7S,7aS)-methyl 5,7-dihydroxy-7-methyl-1-((2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yloxy)-1,4a,5,6,7,7a-hexahydrocyclopenta[c]pyran-4-carboxylate
- METHYL (1S,4AS,5R,7S,7AS)-5,7-DIHYDROXY-7-METHYL-1-{[(2S,3R,4S,5S,6R)-3,4,5-TRIHYDROXY-6-(HYDROXYMETHYL)OXAN-2-YL]OXY}-1H,4AH,5H,6H,7AH-CYCLOPENTA[C]PYRAN-4-CARBOXYLATE
149.0483 100
177.0454 85.17
121.0535 53.92
121.0592 33.33
79.0512 29.98
209.0638 100
191.0569 93.71
149.0453 87.55
177.0403 58.98
195.0534 55.93
243.085712 0.54
101.022621 0.25
59.012317 0.09
405.139576 0.04
225.075122 0.04
91.05672658037105 0.08
77.03963958037104 0.08
65.03923458037104 0.07
115.05484458037104 0.04
128.06223858037106 0.03
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=KKSYAZCUYVRKML-IRDZEPHTSA-N
- ChEBIShanzhiside methyl esterhttps://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:193584
- LOTUS - the natural products occurrence databaseLICENSEThe code for LOTUS is released under the GNU General Public License v3.0.https://lotus.nprod.net/Shanzhiside methyl esterhttps://www.wikidata.org/wiki/Q104252333LOTUS Treehttps://lotus.naturalproducts.net/
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.htmlChEMBL Protein Target Treehttps://www.ebi.ac.uk/chembl/g/#browse/targets
- Japan Chemical Substance Dictionary (Nikkaji)
- Natural Product Activity and Species Source (NPASS)Shanzhiside Methyl Esterhttps://bidd.group/NPASS/compound.php?compoundID=NPC148270
- MassBank of North America (MoNA)LICENSEThe content of the MoNA database is licensed under CC BY 4.0.https://mona.fiehnlab.ucdavis.edu/documentation/license
- Metabolomics WorkbenchShanzhiside Methyl Esterhttps://www.metabolomicsworkbench.org/data/StructureData.php?RegNo=121733
- SpectraBase#6;SHANSHISIDE-METHYLESTER;5,7-DIHYDROXY-7-METHYL-1-(3,4,5-TRIHYDROXY-6-HYDROXYMETHYL)-TETRAHYDRO-2H-PYRAN-2-YL-OXY)-1,4A,5,6,7,7A-HEXAHYDROCYCLOPENTA-[C]-PYRAhttps://spectrabase.com/spectrum/B9GtLyUc2cX
- Springer Nature
- WikidataShanzhiside methyl esterhttps://www.wikidata.org/wiki/Q104252333
- PubChem
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/NORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 396193791https://pubchem.ncbi.nlm.nih.gov/substance/396193791