Scorpion
PubChem CID
197701
Molecular Formula
Synonyms
- MTI-446
- CHEBI:39183
- Guanidine, N''-methyl-N-nitro-N'-[(tetrahydro-3-furanyl)methyl]-
- 1-Methyl-3-nitro-2-[(tetrahydrofuran-3-yl)methyl]guanidine
- Scorpion
Molecular Weight
202.21 g/mol
Computed by PubChem 2.2 (PubChem release 2024.11.20)
Dates
- Create:2005-08-09
- Modify:2025-01-11
Description
Scorpion has been reported in Streptomyces canus with data available.
Chemical Structure Depiction

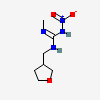
2-methyl-1-nitro-3-(oxolan-3-ylmethyl)guanidine
Computed by Lexichem TK 2.7.0 (PubChem release 2024.11.20)
InChI=1S/C7H14N4O3/c1-8-7(10-11(12)13)9-4-6-2-3-14-5-6/h6H,2-5H2,1H3,(H2,8,9,10)
Computed by InChI 1.07.0 (PubChem release 2024.11.20)
YKBZOVFACRVRJN-UHFFFAOYSA-N
Computed by InChI 1.07.0 (PubChem release 2024.11.20)
CN=C(NCC1CCOC1)N[N+](=O)[O-]
Computed by OEChem 2.3.0 (PubChem release 2024.12.12)
C7H14N4O3
Computed by PubChem 2.2 (PubChem release 2024.11.20)
222540-72-9
- 1-methyl-2-nitro-3-((3-tetrahydrofuryl)methyl)guanidine
- dinotefuran
- MTI 446
- MTI-446
- N''-methyl-N-nitro-N'-((tetrahydro-3-furanyl)methyl)guanidine
- MTI-446
- CHEBI:39183
- Guanidine, N''-methyl-N-nitro-N'-[(tetrahydro-3-furanyl)methyl]-
- 1-Methyl-3-nitro-2-[(tetrahydrofuran-3-yl)methyl]guanidine
- Scorpion
- Venom [Insecticide]
- Safari [Insecticide]
- HSDB 7465
- UNII-1W509710WF
- MFCD06795001
- Dinotefuran (Standard)
- Dinotefuran 10 microg/mL in Acetonitrile
- 2-methyl-1-nitro-3-(oxolan-3-ylmethyl)guanidine
- CHEMBL2228155
- HY-B0827R
- BCP12994
- HY-B0827
- BDBM50486222
- CS-5166
- D5560
- NS00000524
- C18509
- Dinotefuran, PESTANAL(R), analytical standard
- EN300-7399621
- Q3028468
- N-methyl-N'-nitro-N''-[(oxolan-3-yl)methyl]guanidine
- N-methyl-N'-nitro-N''-[(tetrahydro-3-furanyl)methyl]guanidine
- Guanidine, N-methyl-N'-nitro-N''-((tetrahydro-3-furanyl)methyl)-
Property Name
Property Value
Reference
Property Name
Molecular Weight
Property Value
202.21 g/mol
Reference
Computed by PubChem 2.2 (PubChem release 2024.11.20)
Property Name
XLogP3-AA
Property Value
0
Reference
Computed by XLogP3 3.0 (PubChem release 2024.11.20)
Property Name
Hydrogen Bond Donor Count
Property Value
2
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2024.11.20)
Property Name
Hydrogen Bond Acceptor Count
Property Value
4
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2024.11.20)
Property Name
Rotatable Bond Count
Property Value
4
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2024.11.20)
Property Name
Exact Mass
Property Value
202.10659032 Da
Reference
Computed by PubChem 2.2 (PubChem release 2024.11.20)
Property Name
Monoisotopic Mass
Property Value
202.10659032 Da
Reference
Computed by PubChem 2.2 (PubChem release 2024.11.20)
Property Name
Topological Polar Surface Area
Property Value
91.5 Ų
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2024.11.20)
Property Name
Heavy Atom Count
Property Value
14
Reference
Computed by PubChem
Property Name
Formal Charge
Property Value
0
Reference
Computed by PubChem
Property Name
Complexity
Property Value
225
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2024.11.20)
Property Name
Isotope Atom Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Atom Stereocenter Count
Property Value
1
Reference
Computed by PubChem
Property Name
Defined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Covalently-Bonded Unit Count
Property Value
1
Reference
Computed by PubChem
Property Name
Compound Is Canonicalized
Property Value
Yes
Reference
Computed by PubChem (release 2021.10.14)
Pharmaceuticals -> UK Veterinary Medicines Directorate List
S104 | UKVETMED | UK Veterinary Medicines Directorate's List | DOI:10.5281/zenodo.7802119
Spectra ID
Instrument Type
LC-ESI-QFT
Ionization Mode
positive
Top 5 Peaks
129.0896 27.87
114.1024 14.56
113.0947 13.54
87.079 10.98
100.0868 5.40
Notes
adduct_type [M+H]+ original_collision_energy 15 (nominal) CannabisDB pesticides spectra from Mona 2020 August Q Exactive Orbitrap Thermo Scientific
Spectra ID
Instrument Type
LC-ESI-QFT
Ionization Mode
positive
Top 5 Peaks
129.0896 28.29
114.1024 14.93
87.079 13.30
113.0947 11.65
100.0869 7.61
Notes
adduct_type [M+H]+ original_collision_energy 30 (nominal) CannabisDB pesticides spectra from Mona 2020 August Q Exactive Orbitrap Thermo Scientific
Accession ID
Authors
Beck B, Stravs M, Schymanski E, Singer H, Department of Environmental Chemistry, Eawag
Instrument
Q Exactive Orbitrap Thermo Scientific
Instrument Type
LC-ESI-QFT
MS Level
MS2
Ionization Mode
POSITIVE
Ionization
ESI
Collision Energy
15 (nominal)
Fragmentation Mode
HCD
Column Name
XBridge C18 3.5um, 2.1x50mm, Waters
Retention Time
2.7 min
Precursor m/z
203.1139
Precursor Adduct
[M+H]+
Top 5 Peaks
129.0896 999
114.1024 521
113.0947 485
87.079 393
100.0868 193
License
CC BY
Accession ID
Authors
Beck B, Stravs M, Schymanski E, Singer H, Department of Environmental Chemistry, Eawag
Instrument
Q Exactive Orbitrap Thermo Scientific
Instrument Type
LC-ESI-QFT
MS Level
MS2
Ionization Mode
POSITIVE
Ionization
ESI
Collision Energy
30 (nominal)
Fragmentation Mode
HCD
Column Name
XBridge C18 3.5um, 2.1x50mm, Waters
Retention Time
2.7 min
Precursor m/z
203.1139
Precursor Adduct
[M+H]+
Top 5 Peaks
129.0896 999
114.1024 526
87.079 469
113.0947 411
100.0869 268
License
CC BY
Follow these links to do a live 2D search or do a live 3D search for this compound, sorted by annotation score. This section is deprecated (see here for details), but these live search links provide equivalent functionality to the table that was previously shown here.
Same Connectivity Count
Same Stereo Count
Same Isotope Count
Same Parent, Connectivity Count
Same Parent, Stereo Count
Same Parent, Isotope Count
Same Parent, Exact Count
Mixtures, Components, and Neutralized Forms Count
Similar Compounds (2D)
Similar Conformers (3D)
PubMed Count
Insecticides
Pesticides designed to control insects that are harmful to man. The insects may be directly harmful, as those acting as disease vectors, or indirectly harmful, as destroyers of crops, food products, or textile fabrics. (See all compounds classified as Insecticides.)
The LOTUS Initiative for Open Natural Products Research: frozen dataset union wikidata (with metadata) | DOI:10.5281/zenodo.5794106
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/
- ChemIDplusChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.htmlChEMBL Protein Target Treehttps://www.ebi.ac.uk/chembl/g/#browse/targets
- Human Metabolome Database (HMDB)LICENSEHMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications.http://www.hmdb.ca/citing1-Methyl-2-nitro-3-((tetrahydrofuran-3-yl)methyl)guanidinehttp://www.hmdb.ca/metabolites/HMDB0244741HMDB0244741_msms_1479760https://hmdb.ca/metabolites/HMDB0244741#spectra
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.htmlAnimal drugs in Japanhttp://www.genome.jp/kegg-bin/get_htext?br08331.keg
- LOTUS - the natural products occurrence databaseLICENSEThe code for LOTUS is released under the GNU General Public License v3.0.https://lotus.nprod.net/LOTUS Treehttps://lotus.naturalproducts.net/
- MassBank Europe
- MassBank of North America (MoNA)LICENSEThe content of the MoNA database is licensed under CC BY 4.0.https://mona.fiehnlab.ucdavis.edu/documentation/license
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/DinotefuranNORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- Springer Nature
- Wikidatadinotefuranhttps://www.wikidata.org/wiki/Q3028468
- PubChem
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmldinotefuranhttps://www.ncbi.nlm.nih.gov/mesh/67465368Insecticideshttps://www.ncbi.nlm.nih.gov/mesh/68007306
- EPA Substance Registry ServicesEPA SRS List Classificationhttps://sor.epa.gov/sor_internet/registry/substreg/LandingPage.do
CONTENTS