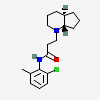
Rodocaine
PubChem CID
71441
Molecular Formula
Synonyms
- Rodocaine
- 38821-80-6
- 3-[(4aR,7aS)-2,3,4,4a,5,6,7,7a-octahydrocyclopenta[b]pyridin-1-yl]-N-(2-chloro-6-methylphenyl)propanamide
- R 19,317
- 9W0Z08C70V
Molecular Weight
320.9 g/mol
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Dates
- Create:2005-08-08
- Modify:2025-01-04
Chemical Structure Depiction
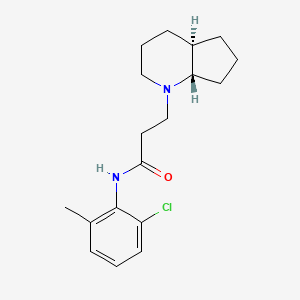
3-[(4aR,7aS)-2,3,4,4a,5,6,7,7a-octahydrocyclopenta[b]pyridin-1-yl]-N-(2-chloro-6-methylphenyl)propanamide
Computed by Lexichem TK 2.7.0 (PubChem release 2021.10.14)
InChI=1S/C18H25ClN2O/c1-13-5-2-8-15(19)18(13)20-17(22)10-12-21-11-4-7-14-6-3-9-16(14)21/h2,5,8,14,16H,3-4,6-7,9-12H2,1H3,(H,20,22)/t14-,16+/m1/s1
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
ICLIXBRUSBYXEV-ZBFHGGJFSA-N
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
CC1=C(C(=CC=C1)Cl)NC(=O)CCN2CCC[C@@H]3[C@@H]2CCC3
Computed by OEChem 2.3.0 (PubChem release 2024.12.12)
C18H25ClN2O
Computed by PubChem 2.2 (PubChem release 2021.10.14)
- R 22700
- rodocaine
- rodocaine, (trans)-(+)-isomer
- rodocaine, (trans)-(+-)-isomer
- rodocaine, (trans)-(-)-isomer
- rodocaine, hydrochloride, (trans)-isomer
- rodocaine, monohydrochloride, (cis)-isomer
- trans-6-chloro-2,3,4,4a,5,6,7,7a-octahydro-1H-cyclopenta(b)pyridine-1-propiono-o- toluidide
- Rodocaine
- 38821-80-6
- 3-[(4aR,7aS)-2,3,4,4a,5,6,7,7a-octahydrocyclopenta[b]pyridin-1-yl]-N-(2-chloro-6-methylphenyl)propanamide
- R 19,317
- 9W0Z08C70V
- Rodocaine (USAN)
- trans-6'-Chloro-2,3,4,4a,5,6,7,7a-octahydro-1H-1-pyrindine-1-propiono-o-toluidide
- RODOCAINE [USAN]
- 1H-Pyrindine-1-propanamide, N-(2-chloro-6-methylphenyl)octahydro- trans-
- Rodocainum
- Rodocaina
- Rodocaine [USAN:INN]
- Rodocainum [INN-Latin]
- Rodocaina [INN-Spanish]
- Rodocain
- UNII-9W0Z08C70V
- R 22,700 [AS HYDROCHLORIDE]
- R19317
- RODOCAINE [INN]
- R-19317
- 2'-Chlor-6'-methyl-3-(perhydrocyclopenta(b)pyridin-1-yl)propionanilid
- trans-N-(2-Chloro-6-methylphenyl)octahydro-1H-1-pyrindine-1-propanamide
- trans-(+)-N-(2-Chloro-6-methylphenyl)octahydro-1H-1-pyrindine-1-propanamide
- 1H-1-Pyrindine-1-propanamide, N-(2-chloro-6-methylphenyl)octahydro-, trans-(+)-
- SCHEMBL591370
- CHEMBL2105238
- DTXSID501043298
- AKOS040753807
- trans-(-)-N-(2-Chloro-6-methylphenyl)octahydro-1H-1-pyrindine-1-propanamide
- 1H-1-Pyrindine-1-propanamide, N-(2-chloro-6-methylphenyl)octahydro-, trans-(-)-
- 39489-97-9
- NS00122164
- D05743
- R 22700
- Q27273297
- 39489-99-1
Property Name
Property Value
Reference
Property Name
Molecular Weight
Property Value
320.9 g/mol
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
XLogP3-AA
Property Value
3.7
Reference
Computed by XLogP3 3.0 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Donor Count
Property Value
1
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Acceptor Count
Property Value
2
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Rotatable Bond Count
Property Value
4
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Exact Mass
Property Value
320.1655411 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Monoisotopic Mass
Property Value
320.1655411 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Topological Polar Surface Area
Property Value
32.3 Ų
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Heavy Atom Count
Property Value
22
Reference
Computed by PubChem
Property Name
Formal Charge
Property Value
0
Reference
Computed by PubChem
Property Name
Complexity
Property Value
389
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Isotope Atom Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Atom Stereocenter Count
Property Value
2
Reference
Computed by PubChem
Property Name
Undefined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Covalently-Bonded Unit Count
Property Value
1
Reference
Computed by PubChem
Property Name
Compound Is Canonicalized
Property Value
Yes
Reference
Computed by PubChem (release 2021.10.14)
Follow these links to do a live 2D search or do a live 3D search for this compound, sorted by annotation score. This section is deprecated (see here for details), but these live search links provide equivalent functionality to the table that was previously shown here.
Same Connectivity Count
Same Parent, Connectivity Count
Same Parent, Exact Count
Mixtures, Components, and Neutralized Forms Count
Similar Compounds (2D)
Similar Conformers (3D)
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=ICLIXBRUSBYXEV-ZBFHGGJFSA-N
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/
- ChemIDplusRodocaine [USAN:INN]https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=00388218061H-1-Pyrindine-1-propanamide, N-(2-chloro-6-methylphenyl)octahydro-, trans-(+)-https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=00394899791H-1-Pyrindine-1-propanamide, N-(2-chloro-6-methylphenyl)octahydro-, trans-(-)-https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0039489991ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- EPA DSSToxCompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.html
- Japan Chemical Substance Dictionary (Nikkaji)
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.html
- NCI Thesaurus (NCIt)LICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuseNCI Thesaurushttps://ncit.nci.nih.gov
- Springer Nature
- Wikidata
- PubChem
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.html
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/NORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 388327503https://pubchem.ncbi.nlm.nih.gov/substance/388327503
CONTENTS