(+)-Coclaurine
PubChem CID
440989
Molecular Formula
Synonyms
- (+)-Coclaurine
- (R)-Coclaurine
- 2196-60-3
- d-Coclaurine
- Sanjoinine K
Molecular Weight
285.34 g/mol
Computed by PubChem 2.1 (PubChem release 2021.05.07)
Dates
- Create:2005-06-24
- Modify:2025-01-18
Description
(R)-coclaurine is a coclaurine. It is an enantiomer of a (S)-coclaurine.
(R)-Coclaurine has been reported in Stephania cephalantha, Cyclea barbata, and other organisms with data available.
Chemical Structure Depiction
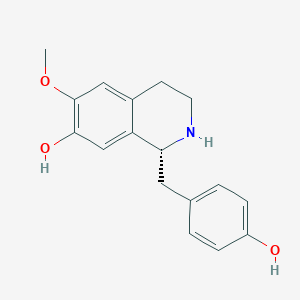
(1R)-1-[(4-hydroxyphenyl)methyl]-6-methoxy-1,2,3,4-tetrahydroisoquinolin-7-ol
Computed by LexiChem 2.6.6 (PubChem release 2019.06.18)
InChI=1S/C17H19NO3/c1-21-17-9-12-6-7-18-15(14(12)10-16(17)20)8-11-2-4-13(19)5-3-11/h2-5,9-10,15,18-20H,6-8H2,1H3/t15-/m1/s1
Computed by InChI 1.0.5 (PubChem release 2019.06.18)
LVVKXRQZSRUVPY-OAHLLOKOSA-N
Computed by InChI 1.0.5 (PubChem release 2019.06.18)
COC1=C(C=C2[C@H](NCCC2=C1)CC3=CC=C(C=C3)O)O
Computed by OEChem 2.3.0 (PubChem release 2024.12.12)
C17H19NO3
Computed by PubChem 2.1 (PubChem release 2019.06.18)
- (+)-Coclaurine
- (R)-Coclaurine
- 2196-60-3
- d-Coclaurine
- Sanjoinine K
- (R)-d-Coclaurine
- CHEMBL256448
- CHEBI:27482
- (1R)-1-[(4-hydroxyphenyl)methyl]-6-methoxy-1,2,3,4-tetrahydroisoquinolin-7-ol
- (1R)-1-(4-hydroxybenzyl)-6-methoxy-1,2,3,4-tetrahydroisoquinolin-7-ol
- 7-Isoquinolinol, 1,2,3,4-tetrahydro-1-((4-hydroxyphenyl)methyl)-6-methoxy-, (R)-
- 7-ISOQUINOLINOL,1,2,3,4-TETRAHYDRO-1-[(4-HYDROXYPHENYL)METHYL]-6-METHOXY-, (1R)-
- (R)-1,2,3,4-Tetrahydro-1-[(4-hydroxyphenyl)methyl]-6-methoxy-7-isoquinolinol
- 6-Methoxy-7-hydroxy-(1R)-[(4-hydroxyphenyl)methyl]-1,2,3,4-tetrahydroisoquinoline
- (+)-R-Coclaurine
- (+)-1R-Cocluaurine
- (R)-(+)-coclaurine
- (+)-(R)-Coclaurine
- (+)-1(R)-Coclaurine
- SCHEMBL21008643
- DTXSID70176367
- HY-N2550
- BDBM50241488
- AKOS040758806
- 7-Isoquinolinol, 1,2,3,4-tetrahydro-1-[(4-hydroxyphenyl)methyl]-6-methoxy-, (1R)-
- AC-34837
- DA-59429
- TS-09149
- CS-0022822
- C06349
- E87186
- Q27103156
Property Name
Property Value
Reference
Property Name
Molecular Weight
Property Value
285.34 g/mol
Reference
Computed by PubChem 2.1 (PubChem release 2021.05.07)
Property Name
XLogP3-AA
Property Value
2.6
Reference
Computed by XLogP3 3.0 (PubChem release 2019.06.18)
Property Name
Hydrogen Bond Donor Count
Property Value
3
Reference
Computed by Cactvs 3.4.6.11 (PubChem release 2019.06.18)
Property Name
Hydrogen Bond Acceptor Count
Property Value
4
Reference
Computed by Cactvs 3.4.6.11 (PubChem release 2019.06.18)
Property Name
Rotatable Bond Count
Property Value
3
Reference
Computed by Cactvs 3.4.6.11 (PubChem release 2019.06.18)
Property Name
Exact Mass
Property Value
285.13649347 Da
Reference
Computed by PubChem 2.1 (PubChem release 2021.05.07)
Property Name
Monoisotopic Mass
Property Value
285.13649347 Da
Reference
Computed by PubChem 2.1 (PubChem release 2021.05.07)
Property Name
Topological Polar Surface Area
Property Value
61.7 Ų
Reference
Computed by Cactvs 3.4.6.11 (PubChem release 2019.06.18)
Property Name
Heavy Atom Count
Property Value
21
Reference
Computed by PubChem
Property Name
Formal Charge
Property Value
0
Reference
Computed by PubChem
Property Name
Complexity
Property Value
330
Reference
Computed by Cactvs 3.4.6.11 (PubChem release 2019.06.18)
Property Name
Isotope Atom Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Atom Stereocenter Count
Property Value
1
Reference
Computed by PubChem
Property Name
Undefined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Covalently-Bonded Unit Count
Property Value
1
Reference
Computed by PubChem
Property Name
Compound Is Canonicalized
Property Value
Yes
Reference
Computed by PubChem (release 2019.01.04)
MoNA ID
MS Category
Experimental
MS Type
Other
MS Level
MS2
Precursor Type
[M+H]+
Precursor m/z
286.1447048359982
Ionization Mode
positive
Retention Time
1.94
Top 5 Peaks
107.04931653159674 100
115.05461780944785 9.11
143.04924643486927 5.66
269.1200307458231 5.07
237.0954005200366 4.78
MoNA ID
MS Category
Experimental
MS Type
Other
Precursor Type
[M+H]+
Top 5 Peaks
107.04928588867188 67838992
269.11767578125 23602484
286.14410400390625 12730624
175.07559204101562 9290979
237.0913848876953 7067010.50
Follow these links to do a live 2D search or do a live 3D search for this compound, sorted by annotation score. This section is deprecated (see here for details), but these live search links provide equivalent functionality to the table that was previously shown here.
Same Connectivity Count
Same Parent, Connectivity Count
Same Parent, Exact Count
Mixtures, Components, and Neutralized Forms Count
Similar Compounds (2D)
Similar Conformers (3D)
PubMed Count
The LOTUS Initiative for Open Natural Products Research: frozen dataset union wikidata (with metadata) | DOI:10.5281/zenodo.5794106
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/(+)-Coclaurinehttps://commonchemistry.cas.org/detail?cas_rn=2196-60-3
- ChemIDplusChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- EPA DSSTox7-Isoquinolinol, 1,2,3,4-tetrahydro-1-((4-hydroxyphenyl)methyl)-6-methoxy-, (R)-https://comptox.epa.gov/dashboard/DTXSID70176367CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- ChEBI
- LOTUS - the natural products occurrence databaseLICENSEThe code for LOTUS is released under the GNU General Public License v3.0.https://lotus.nprod.net/(R)-Coclaurinehttps://www.wikidata.org/wiki/Q27103156LOTUS Treehttps://lotus.naturalproducts.net/
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.htmlChEMBL Protein Target Treehttps://www.ebi.ac.uk/chembl/g/#browse/targets
- Japan Chemical Substance Dictionary (Nikkaji)
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.html
- Natural Product Activity and Species Source (NPASS)(R)-(+)-Coclaurinehttps://bidd.group/NPASS/compound.php?compoundID=NPC76213
- MassBank of North America (MoNA)LICENSEThe content of the MoNA database is licensed under CC BY 4.0.https://mona.fiehnlab.ucdavis.edu/documentation/license
- Metabolomics Workbench
- Therapeutic Target Database (TTD)(R)-(+)-coclaurinehttps://idrblab.net/ttd/data/drug/details/D05OHF
- Thieme ChemistryLICENSEThe Thieme Chemistry contribution within PubChem is provided under a CC-BY-NC-ND 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc-nd/4.0/
- Wikidata(R)-coclaurinehttps://www.wikidata.org/wiki/Q27103156
- PubChem
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- NCBI
CONTENTS